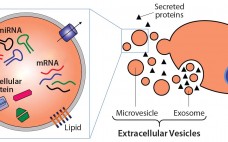
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…
Author Archives: David A. Brindley
The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based Immunotherapies
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology. The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies…
Cell Therapy Bioprocessing Technologies and Indicators of Technological Convergence
The cell therapy industry is undergoing a natural evolution from scientific curiosity into a commercially and clinically attractive opportunity (1). This evolution is by no means complete, and growing evidence suggests that its progression is driving significant developments in cell therapy bioprocessing — notably, convergence. Table 1: 194; () Progressively, bioprocessing technologies primarily used in production of noncell-based products are being evaluated for cell therapy bioprocessing applications (2). Consequently, this process of convergence is leading to an increasing proportion of…
Automation of Cell Therapy Biomanufacturing
Biomanufacturing automation is an established mission-critical step in the commercialization pathway for conventional therapeutics, including small molecules and monoclonal antibodies (MAbs) (1). The prospect of a potential biologic progressing into late-stage clinical trials without a robust biomanufacturing strategy to support at least pilot-plant scale bioprocessing is simply unthinkable. Conversely, the cell therapy industry (or at least a significant proportion of it) regard this as a trend that is unlikely to be mirrored as the industry develops. The aim of this…
Streamlining Cell Therapy Manufacture
The cell therapy industry (CTI) is no longer a cottage industry; it is a distinct and sustainable component of the global healthcare sector (1). Today, CTI prospects are strong, with annual revenues exceeding US$1 billion/year, supported by improving investor sentiment and public support (1,–3). The next phase of CTI growth — toward a multibillion-dollar global industry — will depend on the biomanufacturing community innovating to meet growing market demands and providing products at affordable costs to healthcare payers.…