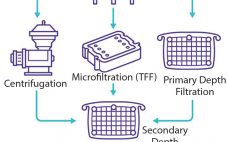
A general vaccine purification strategy can be divided into three stages, with one or more steps for each stage. The first stage is to concentrate and isolate the target molecule quickly to remove it from conditions that could lead to its inactivation or loss. Intermediate purification seeks to remove remaining contaminants, typically using an orthogonal approach. That is followed by a polishing step in which trace impurities are removed through high-efficiency steps because those impurities usually are similar to the…
Author Archives: Priyabrata Pattnaik
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Membrane-Based Clarification of Polysaccharide Vaccines
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1). Polysaccharides are relatively complex carbohydrates made up of many…
Best Practices for Critical Sterile Filter Operation: A Case Study
A number of regulatory guidelines recommend preuse integrity testing of critical sterilizing liquid filters for aseptic processing (1–3). Before sterilization, a preuse test will confirm that a filter is installed properly and was not damaged during shipment or handling. Performing a preuse test after sterilization detects damage that may have occurred during the sterilization cycle. Testing after sterilization limits risk, so it is a practice applied based on risk assessment. Because it is perceived to reduce business loss risk, preuse…
Integrity Testing of Ultrafiltration Systems for Biopharmaceutical Applications
Ultrafiltration (UF) is a membrane-based separation technology commonly used in the biopharmaceutical industry for concentration or diafiltration of protein solutions to remove low–molecular-weight (LMW) impurities or exchange buffers. The nominal MW limit of UF membranes ranges from 1,000 Da (1 kDa) to 1,000,000 Da (1,000 kDa). A target product is retained by the membrane while lower-MW solutes or impurities pass through (1). For a target product with a smaller MW than the impurities, separation is accomplished by allowing…