Current demands placed on the biopharmaceutical industry are pushing manufacturers toward process intensification, an approach that modifies unit operations or an entire manufacturing process to optimize efficiency. Three common intensification scenarios in upstream processing are seed-train intensification (usually at the n – 1 stage), concentrated fed-batch production, and dynamic perfusion (at the production bioreactor stage). In downstream processes, intensification strategies typically involve moving from single- to multicolumn chromatography. Biomanufacturers can realize several kinds of improvements from intensified processing, including reductions…
Author Archives: Gerben Zijlstra
Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…
The 2017 World Biological Forum: Successes and Future Trends in Continuous Biomanufacturing
Continuous biomanufacturing was a central topic at the fourth annual World Biological Forum in Oxford, UK, on 26–28 June 2017. A well-rounded lineup of presenters appeared at this forum held in Oxford University’s Lady Margaret Hall, an eclectic location that well captured the historic charm of the university. Delegates were well supported throughout the meeting with generous meals, refreshments, and assistance provided by helpful staff. Papers were presented in Talbot Hall in the center of the college. The stately main…
How to Balance High-Titer Processes with Consistent Quality?
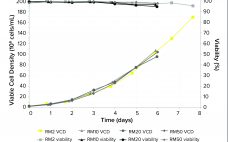
The high cell culture process (XD®) is a continuous process in which both cells and product are retained in a stirred-tank bioreactor using suspension mammalian culture. This is accomplished using a retention system: Fresh medium is continuously supplied, and metabolic byproducts are withdrawn and discarded through the retention system. XD® provides a controlled environment that leads to consistent product quality in terms of bioactivity, glycosylation pattern, and other product characteristics. Moreover, XD® technology is independent…