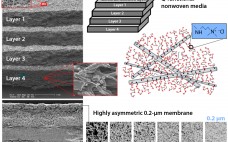
Monoclonal antibodies (MAbs) are the most prominent and successful therapeutic proteins in the pharmaceutical industry. More than 35 MAbs have been approved to treat a range of conditions, and hundreds more are in development (1, 2). Once, the upstream cell culture process was considered the bottleneck to producing high antibody doses required for treatment, but recent advances in cell culture technology have boosted antibody titers to the range of 5–10 g/L (3). That increase in productivity has shifted focus onto…
Sunday November 16, 2025