On Wednesday, 21 June 2017, BioProcess International presented a panel discussion from 1:20 to 2:30 pm as part of the “Emerging Techniques and Technologies” session of its BPI Theater at the BIO annual convention in San Diego. Moderated by Joshua Speidel (managing director of the commercial practice team at Latham Biopharm Group), this panel comprised Diane Retallack (senior director of upstream processing and intellectual property at Pfenex), Rachel Felberbaum (senior director of business development at Protein Sciences Corporation), and William Taylor…
Author Archives: Alison Center
Current Thinking in Viral Safety: Risk Management Protects Patients
BPI’s editor in chief S. Anne Montgomery recently caught up with long-time editorial advisor Hazel Aranha (purification technologies technology expert for Sartorius Stedim Biotech, North America). They discussed a number of topics related to viral safety. Montgomery: What is the current thinking regarding virus-safety assurance in biopharmaceutical manufacturing? How is the industry preventing viral contamination? Aranha: The “holy grail” of viral safety — absolute freedom from extraneous agents or residual pathogenicity — is a myth. That said, biopharmaceutical products have…
Going After a Moving Target: New Production Methods Aid in the Flu Fight
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (1, 2).…
Rapid Development of High-Quality, Robust Mammalian Cell Culture Manufacturing Processes
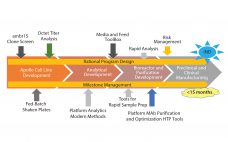
With increasing industry emphasis on providing both rapid and robust processes, companies are reaping the benefits of new tools for risk management and process analytical controls. As a current example of these approaches, Fujifilm Diosynth scientists have accelerated the development process from gene to finish by shortening the timeline, incorporating quality by design (QbD) principles, and designing the process to be as robust as possible. When the Apollo mammalian expression cell line was introduced three years ago, the time from…
Deciding Between Single-Use and Stainless Steel Strategies
A BPI Theater Roundtable at Interphex 2016 On Tuesday, 26 April 2016, Eric S. Langer (managing partner of BioPlan Associates) chaired a morning roundtable titled “Deciding on Single-Use vs. Stainless Steel Strategy: What the CMOs Know That Biopharma Needs” as a follow-up to a similar discussion held last year. Langer brought together a panel of four contract manufacturing organization (CMO) industry experts: Daniel Vellom (senior director of global technology innovation at Sanofi Pasteur) Sue Behrens (senior director of process technology…
Coordination of Single-Use System Standards and Best-Practice Efforts
A BPI Theater Roundtable at Interphex 2016 On Tuesday, 26 April 2016, James D. Vogel (founder and director of The BioProcess Institute at the University of Rhode Island) chaired a midday roundtable titled, “Single-Use Harmonization Town Hall: Coordination of SUS Standards and Best Practice Efforts.” Two industry experts joined him in a panel discussion: Mike Johnson (business development engineering manager for Entegris) Jeff Carter (strategic projects leader at GE Healthcare). All three are members of different groups interested in developing…
Getting the Most in Training for New Technologies
A BPI Theater Roundtable at Interphex 2016 On Wednesday, 27 April 2016, BioProcess International and the Biomanufacturing Training and Education Center (BTEC) at North Carolina State University (Raleigh, NC) presented an afternoon training symposium in the BPI Theater at Interphex 2016. Brian Caine, BPI’s cofounder and publisher, offered some opening remarks: “The purpose of these theaters and BioProcess International magazine is to talk about trends and their combined impact on bioprocessing. With the tremendous amount of new technology and products,…
Industrialization and Commercialization of Gene and Cell Therapies
A BPI Theater Roundtable at the 2016 BIO Convention On Tuesday, 7 June 2016, Mike Ward (global director of content at Informa) chaired an afternoon roundtable titled, “Challenges Associated with the Industrialization and Commercialization of Gene and Cell Therapies.” He brought together a panel of four experts: Morrie Ruffin (managing director, Alliance for Regenerative Medicine, ARM) Michael Werner (ARM’s executive director) Sarah Haecker Meeks (chief scientific officer, Adjuvant Partners; director of technology sections, ARM) Tom Novak (vice president of strategic…
Making the Correct Outsourcing Decisions
A BPI Theater Roundtable at the 2016 BIO Convention On Wednesday, 8 June 2016, Gil Roth (president of the Pharma and Biopharma Outsourcing Association, PBOA) chaired a lunchtime roundtable titled, “Making the Correct Outsourcing Decisions.” He brought together a panel of four experts: Cory Lewis (vice president of business development and marketing at Cook Pharmica) Andrew Sanford (vice president of global business development for biologics at Catalent) David Powell (Pfizer CentreOne) Rajan Puri (senior director of business development at Therapure…