Frozen storage is commonly achieved using single-use containers that enable manufacturing process flexibility and long-term product stability as well as minimize logistics challenges. Single-use containers also are available for storing and transporting frozen products although some require a significant investment, and others don’t offer necessary support and protection.
Frozen storage is commonly achieved using single-use containers that enable manufacturing process flexibility and long-term product stability as well as minimize logistics challenges. Single-use containers also are available for storing and transporting frozen products although some require a significant investment, and others don’t offer necessary support and protection.
Charter Medical recently developed a single-use frozen storage and transport shipping solution designed to complement single-use Freeze-Pak™ STS (FP-STS) biocontainers, which are engineered specifically for frozen storage applications. The bags and shippers are tested for frozen storage and transport to -80°C. FP-STS single-use biocontainers and shippers have been designed to provide a simple solution while delivering the flexibility and durability required for frozen storage and transport.
The purpose of the study presented here was to test the new Freeze-Pak™ STS single-use secondary storage and shipping containers (Charter Medical, Ltd., Winston-Salem, NC) for both storage and transport. Results herein demonstrate that the new single-use STS shipping solution can be used to effectively store and transport Freeze-Pak STS freezing bags to temperatures as low as -80°C.
Methods
FreezeThaw:
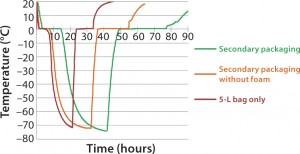
Freeze-Pak™ STS bags (5L) were filled to 80% of nominal capacity and placed into a shipper (Figure 1). They were frozen to -80°C using a chest-style freezer (ULT freezer). Testing with a secondary shipper was performed (5L size, solely as a worst-case scenario) and subsequently evaluated for duration (Figure 2).
Secondary Container Robustness Testing:
A selection of tests were performed to investigate FP-STS secondary storage and transport containers for their intended use.
Handling/Drop Test:
For these tests, bags were filled, assembled in the secondary shipper, and frozen. The containers were removed and immediately dropped from a height of 3 ft. Two separate drops per container were performed (bottom, top, and edge). As a worst-case scenario, 5L bags (five total) were evaluated.
Modified ASTM Transportation Simulation:
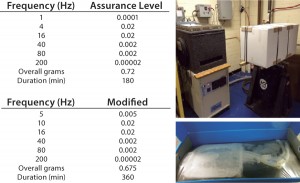
The 5L bags were again evaluated as a worst-case scenario. In brief, bags (in shippers) were frozen to &emdash;80°C and packed into a corrugated external shipping box. Two 5L STS shippers were placed inside the corrugated box with insulation and dry ice to minimize the drop in temperature. The entire box was then dropped five times from 1.5 ft. Subsequently, the container was placed onto a vibration table where transport testing was performed at frequencies shown in Figure 4 for six hours, in accordance with a modified ASTM Assurance Level 1 simulation, followed by a final set of drop tests. Shippers were then removed, thawed at room temperature, and evaluated.
Data Summary
The testing performed in this study is to support the efficacy of the secondary shipper and Freeze-Pak™ STS for use in frozen storage and transport of products. Initial testing was performed to evaluate general freezethaw profiles (Figure 2). As expected, the time to freeze and thaw increased with the addition of the STS shipper.
Next, the shipper was tested to demonstrate the overall capacity to provide protection during normal handling of frozen single-use products. The 5L shippers frozen to -80°C were dropped (as described in Methods above) and then evaluated. Even with this extreme testing, the frozen FP-STS bags remained completely intact and free of any damage (Figure 3). The STS shippers then were evaluated for use in a typical transportation setting. No damage to the frozen 5L FP-STS bags was observed (Figure 4).
Note:
All testing was performed to support Freeze-Pak™ STS bags up to 5L. For FP-STS bags of 10L and 20L, the BioShell offered by UFP Technologies (Georgetown, MA) has been tested for efficiency and use.
A Single-Use Frozen Storage and Transport Solution
The Freeze-Pak™ STS disposable freezing bags and secondary shippers are designed to support frozen storage product needs. The studies herein support the efficacy and durability of the new STS shippers for storage and transport to temperatures as low as -80°C. In conclusion, the single-use Freeze-Pak™ STS primary and secondary containers from Charter Medical, Ltd. offer a new durable yet flexible freezing, storage, and transport solution.
Corresponding author Dominic Clarke, PhD, is the global product manager for bioprocessing and cell therapy (DClarke@Lydall.com).
Brad Bennett is a new product development engineer at Charter Medical, Ltd., 3948-A Westpoint Blvd, Winston-Salem, NC 27103,
www.Chartermedical.com.
Freeze-Pak is a trademark of Charter Medical, Ltd., Winston Salem, NC.