Following the initial observations by Chen et al., leading manufacturers of biopharmaceuticals have determined that endotoxin (LPS) can be “masked” in biopharmaceutical formulations typically containing phosphate, polysorbate, and sugars (1). Being in a masked state, endotoxin is undetectable by the commonly used factor C detection methods such as the Limulus amebocyte lysate test (LAL). This phenomenon has also been denominated low endotoxin recovery (LER).
Following the initial observations by Chen et al., leading manufacturers of biopharmaceuticals have determined that endotoxin (LPS) can be “masked” in biopharmaceutical formulations typically containing phosphate, polysorbate, and sugars (1). Being in a masked state, endotoxin is undetectable by the commonly used factor C detection methods such as the Limulus amebocyte lysate test (LAL). This phenomenon has also been denominated low endotoxin recovery (LER).
As previously shown by Reich et al. (2), endotoxin masking is time dependent, meaning that a defined amount of endotoxin spiked to a sample at time point zero often cannot be recovered (detected) with commonly used methods after hours or days. This is sometimes the case even within minutes of positive product controls (PPCs) initially have indicated a positive test result. Such lack of test reliability can ultimately affect patient safety.
Reich has studied the mechanisms behind endotoxin masking based on previous findings showing that factor C requires endotoxin as aggregates to be activated (3). The interaction of buffer components, surfactants, and the active pharmaceutical ingredient (API) changes the aggregation state of endotoxin, thereby rendering factor C activation difficult, which in turn affects endotoxin recovery. Nakamura et al. observed similar behavior of surfactant-containing samples (4).
Finding the Missing Endotoxin — Development of a Sample Preparation Method for Biopharmaceuticals: Since early 2013, Hyglos has led a dedicated research effort to develop an endotoxin unmasking protocol for affected biopharmaceutical formulations. The project has now resulted in a sample preparation method enabling complete recovery of endotoxin out of affected formulations.
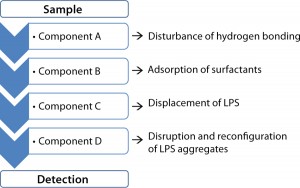
The new method comprises the following four steps: disturbing hydrogen bonding, adsorption of surfactants, displacement of the endotoxin, and change of the endotoxin aggregate form into micelles required for detection (Figure 1). Hyglos offers the needed reagents as a complete sample preparation kit — the Endo-RS® endotoxin recovery kit. The sample preparation kit must be applied in combination with the EndoLISA® endotoxin detection assay, because the solid phase of EndoLISA ensures the complete removal of sample matrix. This advantage of EndoLISA is important because the reagents needed for unmasking the endotoxin would otherwise interfere with homogeneous methods such as LAL.
References
1 Chen J. Low Endotoxin Recovery in Common Biologics Products. 2013 PDA Annual Meeting, Orlando, FL, April 2013.
2 Reich J. Reliability of Endotoxin Detection: Mechanistical Principals of Endotoxin — Masking and Strategies for Demasking. 2014 PDA Europe Pharmaceutical Microbiology Conference, Berlin, February 2014.
3 Mueller M, et al. Aggregates Are the Biologically Active Units of Endotoxin. J. Biolog. Chem. 279(25) 2004: 26307–26313.
4 Nakamura T, et al. Interaction Between Lipopolysaccharide and Intracellular Serine Protease Zymogen, Factor C, from Horseshoe Crab (Tachypleus tridentatus) Hemocytes. J. Biochem. 103, 1988: 370–374.
Johannes Reich is a PhD student at University of Regensburg, Regensburg, Germany. Karolina Heed is director of marketing and sales, and Holger Grallert is vice president of research and development at Hyglos GmbH, Am Neuland 3, 82347 Bernried, Germany; 49-8158-90600, www.hyglos.com.