Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft2 “Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft2) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.
The design includes space for expansion by scale-out as product output demand rises. Segregated suites enable the facility to process different viral vectors simultaneously, including the company’s own LentiVector delivery platform and other types of viral vectors that the company makes through strategic partnerships
as a contract development and manufacturing organization (CDMO).
The processing platform combines a VS pooling stage that applies bioburden control with a VP concentration process that incorporates single-use closed systems followed by automated vial filling and container closure within barrier-isolator technology under full aseptic processing conditions. The filling platform is flexible enough to be configured for filling viral vectors under different conditions, covering a broad range of volumetric concentration factors and vial-size–fill-volume combinations. VS and VP processed within the Oxbox facility are used either for gene transfer in gene-modified autologous or allogeneic cell therapy — or for direct application as in vivo gene-therapy drugs.
| Highlights of the Oxbox Facility
• Filling under barrier-isolator technology with fully integrated, automated filling process equipment operated at positivepressure differentials to support aseptic process operations together with viral containment measures applied to those barriers • 100% inline in-process control (IPC) with weight checks of vials at filling • Minimum product loss in IPC calibration and filling operations • Gloveless filling operations (with automation and intervention-free • Barrier glove access for full qualification and routine environmental monitoring (EM) sampling and for inherent and corrective interventions without compromise to grade A • New methodologies adopted, particularly no-touch transfer (NTT) of ready-to-use (RTU) • Viral clearance by vaporized hydrogen peroxide (VHP) for outer vial surfaces before • VHP residuals biocompatibility managed and qualified through single-use packaging permeation studies and aeration end-points at a nonimpact level |
In BPI’s October featured report, Part 1 of this case study focused on the design of both the facility itself and the formulation and fill–finish (FFF) processes within it (7). Here, we conclude with a discussion of problems solved, decisions made, risk management, and viral safety concerns.
Key Decisions Related to Automation
Oxford Biomedica’s LentiVector platform was developed and qualified using glass vials,with all supporting stability data from experience with that container format as well, so it was to be maintained. Because viral vectors are high value and often developed to treat high-demand unmet patient needs, manufacturers need to maximize the number of suitable units going to patients with minimal loss. Thus, it was decided that 100% in-process control (IPC) weight checks would be required, which ruled out the choice of “nest fillers” that fill vials from nested container packs with statistical IPC applied.
Two measures were applied to reduce product loss. First, Oxford Biomedica implemented an FPC-60 Watson Marlow-Flexicon filling machine that applied IPC calibration (prefilling) without product loss using machine intelligence to “teach” the filler a required filling sequence: fast initially, with a slow top-up to the fill weight. Second, to keep the product fluid path lines as short as possible, the bulk VP bag was moved on an overhead rail inside an F. Ziel GmbH isolator to the point of fill, where a ready-to-use (RtU), presterilized filling needle could be attached.
During the filling process design stage, a key decision to be made was whether to go fully robotic with a gloveless cell that offered some advantages in contamination control. But it was decided that such an approach would have reduced the future flexibility of the system, so an alternative methodology was applied to manage contamination risks in filling through gloveless filling operations.
Gloveless cells have no gloves on their barrier systems and fully rely on robotics for all process manipulations. By contrast, a gloveless-filling operation platform has barrier gloves installed, but they are used only for filling-support activities or environmental monitoring before and after batch-filling operations. Full automation applies without the need for barrier-glove intervention during filling and stoppering of openly exposed sterile containers. If corrective intervention is required during a filling operation (e.g., clearing a stopper- or cap-feed jam), then the process halts automatically when gloves break a light curtain inside the isolator linked to the filling line.
That system provides flexibility to use barrier gloves for closed-barrier interventions that eliminate the need to open the barrier to the surrounding cleanroom environment, thus significantly reducing subsequent in-process product loss. In gloveless-cells operation, such a major open-barrier intrusion for a correction would necessitate a complete restart of manufacturing operations. Oxford Biomedica considered that risk mitigation through gloveless operations was the best option to balance risks, both in processing and interventions, while maintaining full current good manufacturing practice (CGMP) compliance. Both inherent and corrective barrier-glove interventions are part of media fills in aseptic process simulations (APSs).
Through combining a gloveless-operations strategy with barrier technology that incorporates automated filling with fully integrated vaporized hydrogen peroxide (VHP) biodecontamination, all regulatory requirements and expectations are met as specified in European and US guidance. Those include requirements for environmental classification and monitoring with an EU GMP Annex 1 contamination control strategy (CCS). The strategy also includes documenting where quality risk management (QRM) and quality by design (QbD) principles are applied both to facility and process designs and in process operations — and where (justified) alternative methodologies are applied.
Risk Management and Viral Safety
Two contamination risks at the critical point of fill were identified during the design and building phases using computational fluid dynamics (CFD) as illustrated in Figure 5. Primarily, the unidirectional airflow (UDAF) forms a shield around vials to protect the open sterile product. Each vial acts like a dead-leg, so the UDAF at 0.45 m/s downflow does not enter it at that protective velocity. Thus, there is a risk of product contamination if the protective air shield is disturbed around the vial — known as “first-air protection” with clean air from high efficiency particulate (HEPA) air filters. Protective air disturbance and added contamination risk associated with glove interventions highlight the need for automation and gloveless operations.
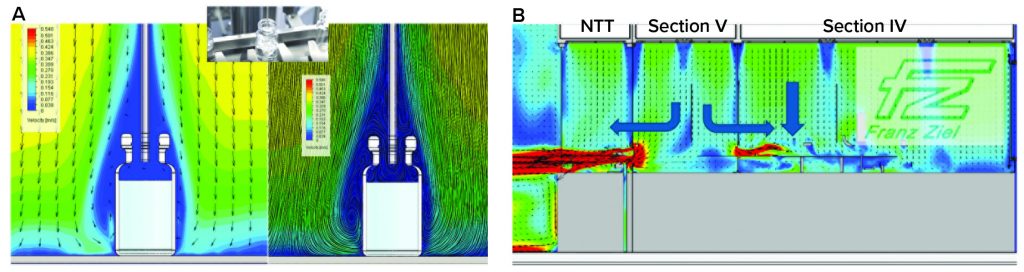
Figure 5: Computational fluid dynamics (a) at the point of fill and (b) at the entry of ready-to-use (RTU) vials into the grade A zone
In addition, it can be seen from the CFD model there is some risk of product being released from a vial during filling by aerosol generation and replacement of vial head-space air by the filling liquid. Because of reduced air velocity in the vial head space, product exiting the vial can contaminate the outer surfaces and present a viral safety issue in subsequent handling. Research studies on a production filling line have confirmed aerosol generation and product release outside vials, which formed a key consideration in Oxford Biomedica’s aseptic-containment strategy (ACS) for viral containment (8).
Rapid Decontamination: In the VP filling process, if even small quantities of a viral vector ended up on the outer surfaces of vials, that would present a viral containment concern and cross-contamination risk along with the potential environmental exposure of a genetically modified organism (GMO). To manage viral containment, after filled vials are integral (stoppers inserted and caps fitted), a selective compliance articulated robot arm (SCARA) machine picks up each vial at the end of the walking-beam filling track and places it into a VHP point-contact exposure basket. Baskets are accumulated as a batch while automated filling continues and then transferred to a rapid decontamination hatch (RDH) that completes a 30-minute rapid VHP cycle to clear potential viral contamination on the outer surfaces of vials before subsequent transfer to visual inspection. To maintain filling operations, empty VHP exposure baskets can be reloaded through the same RDH to receive more filled vials.
No-Touch Transfers: Another part of the viral containment strategy relates to entry of RtU, presterilized containers through the no-touch–transfer (NTT) process that includes “mouse holes” for entry from different-grade NTT zones into the grade A isolator filling zone. Transfers operate at positive-pressure differentials to support aseptic processing and QRM. Typically, air would follow a path from grade A to B to C, from the filling zone in the isolator and out through the mouse holes, potentially exposing a GMO to the environment. To meet requirements for both viral containment and support of aseptic processing for filling, a split-directional air flow was needed in the grade A isolator to prevent air passing from the filling zone to the cleanroom through the NTT pathway. The split air flow was between zones where delidding (Tyvek cover removal from vial trays) takes place and vials are oriented for loading to the filler rotary-feed table for subsequent filling.
The CFD results in Figure 5b show how grade A airflow is contained in those zones. Balance is important, with the incoming horizontal, localized UDAF coming through the mouse hole as it approaches the point of filling. At that critical point, downflow UDAF becomes predominant. This establishes primary protection of the filling process, with first-air protection applied to the open vials while maintaining air segregation from the vial-presentation area. CFD modeling was essential to delivering the required airflow pattern balance before follow-up qualification based on airflow visualization smoke studies.
Further studies were conducted to qualify containment and protection against airborne contamination transfer at those critical mouse-hole interfaces between sections and different GMP grades. Oxford Biomedica used a limitation of risk (LR) particle-challenge method applying particle counters to detect adverse particle transfer that could include microbe-carrying particles (MCPs) (9). This method uses a calculation based on particle-concentration measurement to define a risk factor with associated acceptance criteria to ensure that airborne contamination does not compromise the grade A environment during NTT transfers.
The isolator was designed to provide maximum protection at the critical point-of-filling operation located in a “critical zone” that incorporates the rotary vial-feed table, point of filling (needle and holder), and stopper application devices. In that critical zone, open sterile vials (before filling) and filled vials (before closing) are exposed to the grade A environment.
The Grade A critical zone is protected on one side by mouse-hole barrier airflows and on the other side by an airflow splitting screen/barrier to prevent particulate contamination from the vial-capping and -crimping process. Upon orientation of vials that are ready for loading on the rotary table and their feed onto the filling track, the vial tray protects upright open vials from potential contamination by particle-generating manipulations and transfer steps before entry into the critical zone. Removed vial trays exit away from that zone back through NTTs to packaging-bag and waste management. Vials are fed into the isolator one tray at a time as the feed table is depleted. That enables continuous feeding of vials and an overall reduction of their exposure time in the critical zone, thus reducing contamination risk to the open vials.
In principle, the NTT process relates to semiautomated debagging without user contact. Contents are mechanically ejected from packaging (bags) through a mouse hole into the next higher grade zone. NTT is not just about surface-contamination transfer through debagging, but also about managing airborne contamination transfers at the material entry mouse holes. For quality assurance, qualification studies are required in dynamic operation as vial packs are debagged in successive steps. The first debagging step from grade C to B protects the grade B NTT zone, and the second debagging step from grade B NTT protects the grade A isolator.
The NTT barriers are based on restricted-access barrier system (RABS) principles of airflow protection. But for material transfers, there is no open exposure of sterile vials to the zone environment. The NTT transfer system has been qualified to operate with a grade C background using two debagging steps (C to B to A). Hence, it is directly connected to the filling isolator in the same cleanroom.
Through Oxford Biomedica’s enabling work, RtU presterilized vials prepared for NTT have end-to-end qualification from container manufacturing and sterilization through the supply chain and into point of use. There, the sterile barrier has been qualified to remain sterile on entry to the grade A filling zones, requiring no transfer disinfection step. Thus, NTT with semiautomated RtU vial transfers is fully compatible with viral-vector filling operations.
Viral Clearance: As mentioned in Part 1 (7), VHP is used for viral clearance as a cross-contamination control measure in viral vector manufacturing. As a sporicidal agent, it also plays an important role (together with a cleaning step) in biodecontamination of barrier technologies and enclosed process equipment as part of establishing grade A conditions. For viral vectors, GMP requirements of biodecontamination must be balanced against the impacts on biological products from disinfectant residues/residuals.
With extensive implementation of presterilized single-use (SU) product-contact parts in both formulation and filling steps within isolator technology subjected to VHP, the packaging of those parts required a study to verify their impermeability. Because of the high sensitivity of viral vectors to VHP, the company applied an analytical method based on Amplex Red dye and horseradish peroxidase to detect permeation of H2O2 across the packaging layer at a sensitivity of 10 ppb (10).
Before formulation and filling batch activities, a biodecontamination cycle is applied in the isolator to establish grade A conditions. This cycle includes an aeration phase that is qualified to an end-point residual of 0.1 ppm, a level determined not to affect the exemplar viral vector product. A further VHP decontamination cycle is performed after each batch for viral clearance. The postbatch cycle includes viral clearance in filter plenums above the UDAF HEPA filters and through the complete guided air path in the barrier isolator, with an aeration end-point target of 1 ppm at the occupational exposure limit (OEL). Product biocompatibility was not required at that stage because of protection afforded by the container–closure system. Application of H2O2 vapor with small-flash evaporated molecules was key to selection of this method because the vapors can pass through filter media for complete system decontamination — by contrast with larger aerosolized droplets (dry fog) that have inherent limitations.
Designing Processes and Systems in Parallel
The Oxbox project started with an existing building that was used as a shell in which a purpose-built facility was constructed. This approach offered considerable benefits in reducing project time, providing early access for site staff to work alongside contractors, and establishing on-site Oxford Biomedica teams before finalization. The facility and process designs were based on viral vector manufacturing requirements for flexibility and modularity that offer scale-out potential for future capacity increase.
GMP compliance was considered across all relevant international regulations including EU GMP Annex 1 and FDA guidance for sterile manufacture by aseptic processing — not just the EU GMP for advanced therapies. Design of the filling process, including selection of filling equipment and barrier operations, was key to filling viral vectors while following QRM principles with GMP compliance. Key qualifiers are listed in the “Highlights” box. The CCS and ACS strategies were developed together based on requirements for advanced-therapy manufacturing and full GMP compliance along with viral containment of viral vector GMOs.
The advanced therapy medical products (ATMP) community is realizing significant technical advances in filling operations using advanced barrier technologies, with increased levels of automation to meet batch-size demands and GMP expectations alike. ATMP processes often are complex and time sensitive, so operators are critical to the success of these technical advances. Training of GMP operators in good cleanroom behavior, good aseptic techniques, and procedural controls for aseptic processing applied to sterile product manufacturing is key to achieving the high standards required to deliver these life-changing products safely to patients. A key factor in successfully building and operating ATMP facilities like this one continues to be the training and qualification of staff in GMP principles related to sterile-product manufacturing and QRM.
References
For references 1–6, see Part 1 in BPI’s October featured report.
7 Southam L, Drinkwater JL. Formulation, Fill and Finish of Lentiviral Vectors, Part 1: Case Study in Facility and Process Design. BioProcess Int. 19(10) 2021: S14–S20; .
8 Kranenburg H. Filling Aerosol Generation Studies on an Automated Filling Line. ISPE Aseptic Conference. Vienna, Austria, 2018.
9 Ljungqvist B, Reinmuller B. The LR Method in Critical Areas: Airflow Patterns and the Design of Aseptic Interventions. Pharm. Technol. 28(7) 2004: 46–54; https://www.pharmtech.com/view/lr-method-critical-areas-airflow-patterns-and-design-aseptic-interventions.
10 Yim S. Protecting Biotech Products from Hydrogen Peroxide Vapor (VHP). Seventh International Pharma Compliance Congress, 21–23 May 2013, Madrid, Spain; Informa Connect: Boston, MA.
Leslie Southam is the quality assurance manager of the Oxbox project at Oxford Biomedica Ltd. in Oxford, United Kingdom; 44-1865-783-000; https://www.oxb.com. Based in the United Kingdom, corresponding author James L. Drinkwater is head of GMP compliance for Franz Ziel GmbH and elected chair of the Pharmaceutical and Healthcare Sciences Society (PHSS); james.drinkwater@ziel-gmbh.com; https://www.ziel-gmbh.com.
Tyvek is a registered trademark of Dupont. Amplex is a registered trademark of Thermo Fisher Scientific. LentiVector is a registered trademark of Oxford Biomedica.


