Construction is under way on a hybrid biomanufacturing plant at a site in Bangalore, set to support Biocon’s growing monoclonal antibody (MAb) portfolio.
Indian drugmaker Biocon has a growing pipeline of biological products, including Ogivri, the first biosimilar of Roche’s Herceptin (trastuzumab) to be approved by the US Food and Drug Administration (FDA) last December, and Fulphila, the first US biosimilar of Amgen’s cancer drug Neulasta (pegfilgrastim), approved in June.
Both are marketed by Mylan, but including products developed in this partnership, Biocon claims to have one of the largest portfolio of biosimilars globally with an addressable market size of over US$60 billion (€53 billion).
The facility will be built in Bangalore, India. Image: iStock/Tuomas_Lehtinen
While the firm has a biomanufacturing facility at its site in Bangalore, India to support MAb production, Biocon revealed in its end of year report a second facility at the site is being constructed at a cost of around $200 million, spread over three fiscal years.
Siddharth Mittal, Biocon CFO, told BioProcess Insider the construction of the second MAb facility “is important for us to be able to cater to the unfolding market needs as our products go through clinical development and regulatory approvals in various markets across the globe.”
He added: “The timing of investment is linked to capacity requirement for various products in line with our commercial plans. We believe in creating capacities at the right time and not too early, in order to optimize on the operating expenses and idle time.”
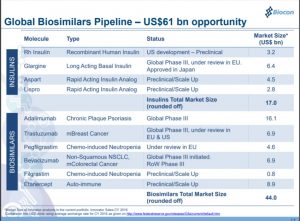
Biocon’s biosimilar pipeline, as of April 2018
The total capacity at the new plant has not been divulged, but Mittal said this is an extension of the hybrid model of using single-use and stainless steel as appropriate to achieve economies of scale and cost, as seen in the first facility.
Once completed, the large-scale drug substance facility will lead to job creation direct and direct for up to 400 people.
For the second quarter fiscal year 2019. Biocon reported biologics sales of 3.7 billion INR ($50 million), up 136% on the same quarter last year.
