The generation of human induced pluripotent stem cells (iPSCs) via reprogramming technology represents a major breakthrough in personalized medicine and the treatment of degenerative diseases. This is mainly because the iPSCs can be expanded in culture and then differentiated into specialized cell types that can be used for clinical applications. Patient-derived iPSCs can be used to model human genetic diseases, produce clinically relevant differentiated cells that display disease pathogenesis, or generate specialized cells through directed differentiation process for autologous cell therapy applications. However, in order to generate high quality, clinically relevant specialized cells for therapeutic procedures, it is necessary to establish a robust and reproducible directed differentiated procedure starting from high quality iPSCs manufactured using a robust and cGMP compliant process. This is a major challenge in enabling clinical utility of iPSC-based therapies partly due to inherent difficulties in achieving high quality cGMP grade iPSCs. Moreover, appropriate cell culture system should be used in the process of cGMP iPSC production to maintain their potential for generation of various functional / specialized cells depending on the target clinical application. Finally, the process of iPSC differentiation into specialized cells is biologically complex and must be carefully developed and optimized to ensure high quality specialized cells can be derived using a robust and well controlled induction process.
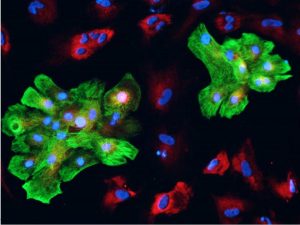 We have previously reported the generation of high quality iPSCs using a cGMP manufacturing process (Baghbaderani et al. 2015) and also reported the detailed characterization of these cells using a wide range of analytical methods including whole genome sequencing (WGS), microarray, and comparative genomic hybridization (aCGH) single nucleotide polymorphism (SNP) analysis (Baghbaderani et al. 2016). These were two critical steps in the development of high quality iPSCs as input material for directed differentiation process. In our most recent publication (Shafa et al. 2017), we have described the clinical potential of the iPSCs generated using our cGMP compatible process by differentiating them into the specialized cells from all three embryonic germ layers including ectoderm, endoderm, and mesoderm. Under controlled induction strategies, the iPSCs have been successfully differentiated into the specialized cells with morphological and cellular characteristics of neural stem cells, definitive endoderm, and cardiomyocytes. We have also demonstrated that our cell culture system (i.e. L7 cell culture system) and iPSCs are not biased toward a specific lineage and that iPSCs can be readily differentiated into the target specialized cells from different lineages. Importantly, we have established robust and reproducible directed differentiation processes by implementing best practices in process optimization. To achieve this goal, after demonstrating the feasibility of inducing cGMP compliant iPSCs into a specific lineage, we identified the gaps in the differentiation process and modified the process by optimizing the critical process parameters. We believe this study highlights a viable proof-of-concept strategy to directly differentiate the same cGMP compliant iPSC lines into the specialized clinically relevant cells from three embryonic lineages and clearly demonstrate the therapeutic potentials of cGMP compliant iPSCs for future clinical applications.
We have previously reported the generation of high quality iPSCs using a cGMP manufacturing process (Baghbaderani et al. 2015) and also reported the detailed characterization of these cells using a wide range of analytical methods including whole genome sequencing (WGS), microarray, and comparative genomic hybridization (aCGH) single nucleotide polymorphism (SNP) analysis (Baghbaderani et al. 2016). These were two critical steps in the development of high quality iPSCs as input material for directed differentiation process. In our most recent publication (Shafa et al. 2017), we have described the clinical potential of the iPSCs generated using our cGMP compatible process by differentiating them into the specialized cells from all three embryonic germ layers including ectoderm, endoderm, and mesoderm. Under controlled induction strategies, the iPSCs have been successfully differentiated into the specialized cells with morphological and cellular characteristics of neural stem cells, definitive endoderm, and cardiomyocytes. We have also demonstrated that our cell culture system (i.e. L7 cell culture system) and iPSCs are not biased toward a specific lineage and that iPSCs can be readily differentiated into the target specialized cells from different lineages. Importantly, we have established robust and reproducible directed differentiation processes by implementing best practices in process optimization. To achieve this goal, after demonstrating the feasibility of inducing cGMP compliant iPSCs into a specific lineage, we identified the gaps in the differentiation process and modified the process by optimizing the critical process parameters. We believe this study highlights a viable proof-of-concept strategy to directly differentiate the same cGMP compliant iPSC lines into the specialized clinically relevant cells from three embryonic lineages and clearly demonstrate the therapeutic potentials of cGMP compliant iPSCs for future clinical applications.
Saturday April 05, 2025
