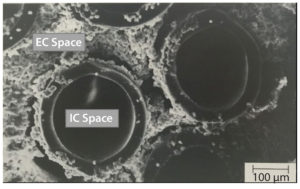
Figure 1: AcuSyst hollow-fiber bioreactors; electron microscopy images of cells growing around hollow fibers within an AcuSyst bioreactor; cells grow to high densities in the extra capillary (EC) space while media flow through the intracapillary (IC) space. These bioreactors scale linearly by adding hollow-fiber cartridges in parallel.
Recent advances in protein engineering have identified new classes of complex biotherapeutics that challenge existing manufacturing platforms. These products have unique cell culture requirements that make them difficult to manufacture cost effectively. Industry standard bioprocessing platforms include large-scale (1,000–5,000 L) batch and fed-batch stirred-tank bioreactors. Historically, the powerhouse molecule of the biologics industry has been human IgG, which necessitates those large-scale platforms. Difficult-to-express proteins and other new modalities (including precision medicine and orphan drugs) have increased pressure on manufacturers to produce smaller batch sizes cost effectively. Traditional stirred tank-bioreactor platforms often cannot meet this need. Cell Culture Company (C3) has developed AcuSyst perfusion bioreactors designed to overcome many limitations imposed by difficult-to-express proteins and small-batch manufacturing. Here we present case studies in which this technology supports high-density mammalian cell culture in a consistent metabolic environment.
Difficult-to-Express Proteins
Complex molecules such as recombinant proteins, bi- and trispecific antibodies, and other unnaturally occurring glycoproteins show increased potential as effective therapeutics. Such proteins often are labeled as “difficult to express” because of low expression titers and sensitivity to environmental conditions. Manufacturers using traditional platforms can face difficulties with these proteins because the cell expression system (and/or the protein) can be particularly sensitive to shear stress or because protein or metabolomics inhibition could suppress protein expression.
Perfusion Bioreactors
Traditional fed-batch bioreactors cannot provide the optimal environment for difficult-to-express proteins. Challenges include the presence of microenvironments, inconsistent nutrient availability, and waste accumulation that can compromise protein production and quality. Standard perfusion technology begins to address those concerns, and AcuSyst perfusion bioreactors are designed to overcome those limitations. The single-use cartridges contain thousands of hollow fibers for culturing and sustaining mammalian cells at very high densities.
Cellulose acetate fibers create two compartments: intracapillary (IC) and extracapillary (EC) spaces. Media flows at high rate through the IC space, and cells grow in the EC space protected from shear forces generated by fluid flow (Figure 1). Media are exchanged across the membrane between the IC and EC spaces through pores in the fibers.
The pores’ ~60-kDa molecular weight cutoff prevents cell and product loss and allows exchange of essential nutrients and removal of waste. Product is continuously harvested from the EC space for regular quality monitoring or downstream processing.
For new biologics making their way through clinical trials, scale-up also can be problematic, especially for a protein that is difficult to express. Revalidation and optimization of manufacturing processes when changing to large tank sizes can be expensive and time consuming. The three designs of the AcuSyst bioreactors (Autovax ID, Maximizer, and Xcellerator) are linearly scalable, enabling fast and efficient scale-up. To increase the size of a culture run, additional single-use hollow fiber cartridges are added in parallel. We reported that each individual cartridge reliably produces the same amount of protein, even in a 10-cartridge system (1). Such predictable scalability saves time and reduces scale-up costs and risks.
Long-Term Protein Production
Many difficult-to-express proteins require a consistent, homeostatic environment for maximized productivity. Cell lines producing low protein titers can require long-term production to express enough product to reach required yields. However, complex biologics and some cell lines can be susceptible to culture environment and thus negatively affected by inconsistent and comparatively turbulent stirred-tank bioreactors. Cell culture process performance also can affect product quality and potency significantly, especially with respect to glycosylation, posttranscriptional modifications, and impurity profiles (2). Therefore, using a biomanufacturing platform that can maintain an optimal environment is crucial to ensuring a high-quality final product.
Perfusion systems can grow healthy cells that produce protein for longer periods than in traditional systems. AcuSyst automated perfusion bioreactors maintain high-density cell health for long-term protein production, with typical runs lasting 60–120 days. By contrast, cell health in a fed-batch tank run declines around 14–21 days (3). Extended cultures enable larger protein harvests from low-producing cell lines with a homeostatic environment.

Figure 2: Consistent metabolic parameters indicate long-term cell health and antibody production in AcuSyst bioreactors. (A) An IgG-producing cell line maintained stable pH conditions throughout the culture. (B) Glucose and lactate values from the same run as in (A); levels remained constant throughout the 40-day culture. (C) Continuous IgG production over six weeks for two cell lines growing in AcuSyst bioreactors — not the same cultures as in (A) and (B).
We tested growth of a hybridoma cell line with relatively low production titers in a 10-cartridge AcuSyst Xcellerator bioreactor for 40 days. The system produced an FDA-approved biologic human IgG, without fully optimized culture parameters. Throughout the run, pH remained stable because of automated monitoring and an integrated control system (Figure 2A). The perfusion system consistently delivers nutrients and removes waste, thus promoting cell health as demonstrated by maintenance of glucose and lactate levels for 40 days (Figure 2B). Such consistent metabolic parameters support findings from other studies showing that perfusion systems can maintain long-term cell health and protein production than batch or fed-batch systems (4, 5).
One advantage of perfusion is the continual harvest of concentrated, cell-free product. An AcuSyst bioreactor’s cell-side EC space contains a relatively small amount of media that can be harvested continuously throughout a culture. An in-line 0.2-µm filter prevents cells and debris from being collected in harvest. Thus, the bioreactor design is such that product never sits in the bioreactor for long, and some downstream steps (e.g., concentration and clarification) can be simplified or eliminated.
In our study, the cell line produced IgG for the duration of the culture (Figure 2C). It is important to note that the bioreactor design essentially eliminates the preinoculation seed train. Each cartridge is seeded with as few as 0.4–0.9 × 106 cells/mL in ~0.2–1 L of cell culture. So production delay observed in Figure 2C is a result of the cells’ growth phase.
Case Studies
Previous studies have suggested that perfusion culture can overcome some limitations of difficult-to-express proteins (6). To test this directly, we grew cells in AcuSyst bioreactors expressing proteins that were known to be challenging to produce in shaker-flask culture. Performance in a shaker-flask environment can predict performance in tanks.
TNF-β Fusion Protein: A TNF-β fusion protein was expressed as 0.023 mg/L in shaker-flask culture. To increase protein titer and to extend the culture run length for maximum productivity, TNF-β protein was produced in a two-cartridge AcuSyst Maximizer bioreactor over a 70-day run consuming 400 L of media. From the EC space, 5 L of supernatant was collected containing 54 mg protein.
Mut-F Recombinant Protein: An anti-HIV-1 replication recombinant protein (Mut-F) was expressed by a Chinese hamster ovary (CHO) cell line in shaker-flask culture at a titer that would be too low for efficient manufacturing (0.012 mg/L). The cell line was grown in a six-cartridge AcuSyst Xcellerator bioreactor for 77 days, consuming 3,850 L of media. A total 220Â mg of Mut-F protein was collected from 15 L of EC space supernatant.
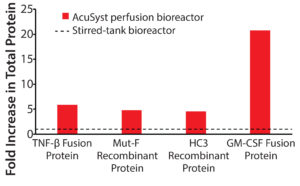
Figure 3: Low-titer proteins produced in AcuSyst bioreactors; data are presented as fold increase in yield for actual culture runs over calculated stirred-tank runs. Relative stirred-tank yields are represented by the dotted line. Stirred-tank yield was calculated using known titer values in spinner flasks and extrapolating up to equivalent media use for bioreactor runs.
HC3 Recombinant Protein: Recombinant protein HC3 expressed by CHO cells suppressed expression at titers >10 mg/L in shaker flasks. We proposed that higher titers might be achieved in a perfusion culture with protein continuously harvested. We grew the cell line in a one-cartridge AcuSyst AutovaxID bioreactor for 30 days, and it consumed a total of 100 L of media. From the EC space, 10 L of supernatant was harvested containing 4.6 total g of HC3 protein.
GM-CSF Fusion Protein: The human–murine chimeric granulocyte-macrophage colony-stimulating factor (GM-CSF) fusion protein was expressed at 0.046 mg/L in shaker-flask culture. It was produced over 30 days in a one-cartridge AcuSyst AutovaxID bioreactor that consumed 200 L of media. From the EC space, 190 total mg of GM-CSF protein was produced in 4 L of supernatant.
Improving Protein Yields
Most biomanufacturing today uses traditional stirred-tank bioreactors. Table 1 lists the actual yield for each protein in an AcuSyst perfusion bioreactor and calculated yields for equivalent media use in a stirred-tank system. Tank yield estimates are based on yields from shaker flasks. For all four proteins, the final titer achieved was significantly higher in perfusion culture than in traditional culture (Figure 3) as was the total amount of protein produced.
Small-Batch Cost Savings
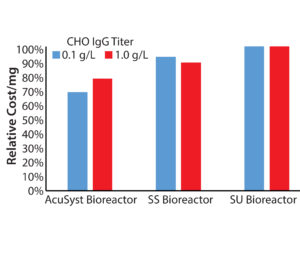
Recent trends toward small batches at clinical and commercial scales have placed a burden on manufacturing organizations to reduce production costs. We calculated the costs associated with producing 200 g of protein from a high-expressing CHO line and a low-expressing CHO line for AcuSyst perfusion with those of stainless steel and single-use stirred tanks (Figure 4).
We assumed a media cost of $30/L for bulk-discounted serum-free CHO media, 30 minutes of labor/day of culture run time, single-use disposable costs and cleaning costs where appropriate, and cost related to the seed train time and materials. For the low-producing (0.1 g/L) cell line, we assumed a run length of 45 days for the AcuSyst bioreactor runs, and 21 days for the tank systems. For the high-producing cell line (1 g/L), we assumed a run length of 21 days for the AcuSyst bioreactor and 12 days for the tank systems. We did not include capital investment or facility overhead in our calculations, but the perfusion bioreactors should benefit there because of their small footprint.
Significant cost savings would be achieved using a perfusion bioreactor for low-producing cell lines (Figure 4). The biggest cost difference comes in the seed train materials and labor: Perfusion bioreactors require ~0.2–1.0 L of inoculum per cartridge, whereas a tank system requires scale-up to ~50% of the final production volume (1).
The Case for Perfusion Technology
New biotherapeutic discoveries are expanding the diversity of biologics and requiring smaller batches of complex biological molecules. Although traditional biomanufacturing platforms produce many kilograms of human IgG, they struggle to produce more complex and smaller batch biologics (milligrams to grams) cost effectively.
AcuSyst perfusion bioreactors meet the needs of manufacturing difficult-to-express proteins. The technology supports long-term cell health and extends the duration of continuous manufacturing runs. Optimized culture environments increase yields of difficult-to-express proteins, enabling production of those that could not be produced cost-effectively in stainless steel and single-use tank bioreactors. The concentrated, cell-free harvest minimizes downstream processing steps. Bioprocessors should consider perfusion technology early in product development to overcome challenges of producing complex biotherapeutics.
References
1 Wozniak E, Biesecker K. Perfusion Solutions for Therapeutic Development. GEN 37(5) 2017.
2 Li F, et al. Cell Culture Processes for Monoclonal Antibody Production. mAbs 2(5) 2010: 466–479.
3 Liu M, et al. Fed-Batch Culture Process Development: Implementing a Novel Nutrient Additive for a Robust, HighTiter, Scalable Process. BioProcess Int. 12(8) 2014: 52–62.
4 Cunha B, et al. Exploring Continuous and Integrated Strategies for the Up- and Downstream Processing of Human Mesenchymal Stem Cells. J. Biotechnol. 213(10) 2015: 97–108; doi: 10.1016/j.jbiotec.2015.02.023.
5 Pollock J, Ho SV, Farid SS. Fed-Batch and Perfusion Culture Processes: Economic, Environmental, and Operational Feasibility under Uncertainty. Biotechnol. Bioeng. 110(1) 2013: 206–219; doi: 10.1002/bit.24608.
6 Warikoo V, et al. Integrated Continuous Production of Recombinant Therapeutics Proteins. Biotechnol. Bioeng. 109(12) 2012: 3018-3029; doi: 10.1002/bit.24584.
Corresponding author Emily Wozniak, PhD, is a project manager, Scott Waniger is vice president of bioprocessing, and Kyle Biesecker, PhD, is a project manager, all at C3 Cell Culture Company, 8500 Evergreen Blvd. NW, Minneapolis, MN 55433; 1-763-786-0302; ewozniak@cellcultureco.com; cellculturecompany.com.

Sounds like interest in hollow-fiber perfusion bioreactors Is growing!
Hi i am interested on small scale cho recombinant human protein of about 340kd 29 disulfide bonds and glucozilations.
Please send me an estimation for pricing offer per gram protein and estimated timeline.
Thanks