The benefits of single-use technologies in both upstream and downstream operations are now widely acknowledged by the biopharmaceutical industry, and have led to radical changes in the design and operation of many bioprocesses. Those changes typically provide more robust processes and increased production flexibility. For mammalian cell culture, cleanable multiuse glass or stainless steel stirred-tank reactors (STRs) have been used successfully for growth of suspension-adapted cell lines in both small- and large-scale systems. However, achieving the same or better performance from a single-use bioreactor presents a significant challenge to product designers and developers (1).
Here we discuss the major design features of a 200-L single-use stirred-tank bioreactor (Pall Allegro STR 200) and present results of studies on its fluid dynamics through measurement of physical parameters that are critical to mammalian cell culture. Our studies confirmed the following:
- High power input from a direct-drive large impeller for fluid volumes up to 200 L
- Rapid and homogeneous mixing in a unique cubical biocontainer with integrated baffles
- Mixing predictions from fluid dynamics computer modeling confirmed by data obtained experimentally
- High oxygen mass-transfer and CO2 stripping rates with ring and open-pipe spargers
- Precise and homogenous control of temperature.
Our results show that single-use technology can be engineered suitably to provide a stirred-tank bioreactor at 200-L scale with fluid dynamics that are suitable for mammalian cell culture. In a future publication, we will present results of studies on cell-culture performance and scalability of this bioreactor using a Chinese hamster ovary (CHO) cell line, comparing Allegro STR 200 performance with that of a 10-L glass, multiuse, stirred-tank bioreactor (2).
Design Features
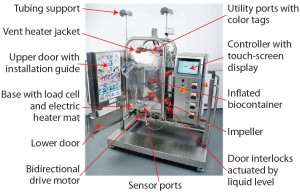
The Pall Allegro STR 200 stirred-tank bioreactor (Figure 1) was developed specifically for mammalian cell culture. Because fully detailed product information is available elsewhere (3), we present here only a short summary to highlight its main design features.
Biocontainer: The fully transparent, flexible biocontainer is cubically shaped with a 60- to 200-L working capacity. Its fluid-contact layer is made of low-density polyethylene with low extractables, as with other Pall Allegro single-use systems. Each biocontainer is integrity tested in manufacturing and supplied with attachments as a single-piece, noninflated assembly. During automated inflation, baffles are formed in the biocontainer walls by protruding metal plates in the surrounding hardware. Direct-drive agitation is provided by a bottom-mounted, three-bladed, pitched “elephant-ear” impeller. The drive is reversible for both up-flow and down-flow operations.
Air and Gas Ports: An aeration port is linked to a ring sparger, and a second port is present for gas overlay. Both are fitted with sterilizing-grade gas filters. An optional open-pipe sparger port provides additional aeration for CO2 stripping. Two vent ports are present for fitment of sterilizing-grade gas filters and external reusable heater jackets to prevent build-up of condensate around the hydrophobic filters.
Liquid Ports: Nine input ports allow addition of liquids such as culture media, nutrients, inoculum, antifoam, and pH stabilizers. Two output ports provide for culture-fluid sampling, and one port is intended for draining.
Sensor Ports: A port at the base of the biocontainer is linked to the temperature sensor. Four additional sensor ports are available for conventional probes such as pH, dissolved oxygen (DO), and CO2. Aseptic insertion is achieved using a presterilized bellows assembly.
Identification of Ports: All utility ports for air, sensors, and liquids are color coded. Tags are numbered in the order of installation to assist users in set-up and connection of the biocontainer to hardware and utilities.
Hardware: The biocontainer is supported by customized stainless steel hardware. Load cells accurately measure liquid input during automated filling and dosing. An electric heater mat on the base provides automated heating and temperature control.
Controls and Data Transfer: A controller enables automated management of every cell culture run. All user interactions happen on a touch-screen interface linked to the controller, with administrator-controlled user rights and passwords. Data are saved in a protected format, and all electronic operations are compatible with the US Code of Federal Regulations Title 21 Part 11. A built-in OPC server provides automated data transfer to any linked supervisory control and data acquisition (SCADA) system during bioreactor operation; data transfer can also be manual using universal serial bus (USB) memory devices.
Investigation of Fluid Dynamics
We investigated the fluid dynamics of a cuboidal STR 200 single-use bioreactor by measuring specific physical parameters known to be critical for mammalian cell culture.
Power Transfer to Fluid: Designs for single-use stirred-tank bioreactors have typically used magnetically coupled drive mechanisms rather than rotating mechanical seals with their risk of leakage. The main disadvantage of such designs is a restriction in power transfer to culture fluids, especially for pilot- and production-scale systems, with consequential limitations on mixing efficiency, mass transfer, temperature control, and other critical operations such as pH control and CO2 stripping. Direct-drive mechanisms can satisfy the full range of power requirements.
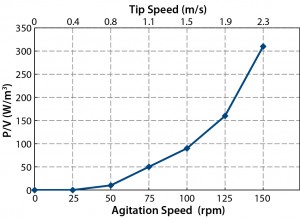
Energy dissipated to fluid by an impeller is the driving force for mixing, but it is also a potential source of stress on sensitive cell lines. An optimal balance must be established between the agitation speed and the sensitivity of a given cell line to hydrodynamic stress. Because different stirred-tank bioreactors have different impeller geometries, agitation speed alone is not a suitable indicator for the hydrodynamic forces generated. Although tip speed takes into account impeller diameter and is regularly used as one indicator of hydrodynamic stress, a more meaningful measurement is power input per volume of fluid (P/V), which is derived from the critical parameters of impeller geometry, agitation speed, and biocontainer volume (4).
We measured the power value (P) using a rotary-torque transducer fitted to the bioreactor shaft between impeller and motor. First, we measured power dissipated by the impeller rotating in air, then subtracted that result from the value obtained with it rotating in 200 L of water at 37 °C to give a true sense of power dissipated to the fluid.
We calculated power values using the following equation: P = 2πNM, in which P is the power value in Watts; N is the agitation speed in rev/s, and M is the torque value in Newton meters (Nm). The tip speed was calculated from πND, in which D is the diameter of impeller. The results (Figure 2) show that high P/V values could be achieved (e.g., 310 W/m3 at an agitation speed of 150 rpm). This capability for high power input when required can particularly benefit mammalian cell cultures in which mixing at high cell densities and viscosities may be essential at some stage in the culture growth cycle.
The potential impact of those power values on the hydrodynamic sensitivity of cells in culture must be taken into account. Impeller tip speed also is one critical factor. Operators often apply a notional tip-speed limit of 1.5 m/s as a general guide for stirred-tank bioreactors. It is generally thought that cell damage may begin above that value (5), even though no substantive evidence supports such a restrictive guideline across all mammalian cell culture processes. For example, Hoeks et al. reported values well in excess of 1.5 m/s in large-scale cell culture (6).
We calculated tip speeds in our power transfer studies (Figure 2). The guideline value of 1.5 m/s corresponded to an agitation speed of 100 rpm. In our CHO cell culture studies (2), we used an agitation speed of 80 rpm to give a P/V value of 55 W/m3 and a calculated tip speed of 1.2 m/s. Under those conditions, we obtained high cell densities and viabilities. Those results demonstrated that the Allegro STR 200 bioreactor could provide high power-transfer capacity and flexibility of power input to meet the variable demands associated with mammalian cell culture applications.
Mixing Efficiency: Together with efficient mixing, good pH and gas control are essential to preventing high concentrations in cell culture. To achieve the required mixing performance, especially for production-scale systems, direct-drive stirred-tank stainless steel reactors have become the preferred design to eliminate solute gradients and ensure good mass transfer.
Another important factor to consider in design of a single-use system is the geometry of the biocontainer. Most stainless steel and glass stirred-tank bioreactors are cylindrical and incorporate baffles to minimize swirling vortices and excessive laminar radial flow, both of which can affect mixing efficiency. But designers of single-use products can consider other geometric forms such as cuboidal shapes because they have no requirement for pressurization to withstand steam-in-place temperatures and pressures. Fluid dynamics of these alternative geometries must be fully investigated, however, so that the design and engineering of such bioreactors can take into account both the powered agitation mechanism and the biocontainer profile to maximize mixing efficiency.
The bioreactor we evaluated was engineered to incorporate the critical features described above. A direct- drive, three-bladed, pitched “elephant ear” impeller (Photo 2) with a 0.5 aspect ratio (impeller diameter to vessel diameter) provides sufficient agitation and power transfer. The unique cubical geometry and built-in baffles assist turbulent flow, with rounded corners to prevent dead zones.
Mixing Times and Homogeneity: Rapid mixing times are essential for adding concentrated nutrients or other chemicals to maintain cell viability and high productivity. These requirements become even more challenging at low agitation speeds and with increasing scale, especially with addition of highly concentrated fluids. Differences in concentration can be damaging to cell growth.
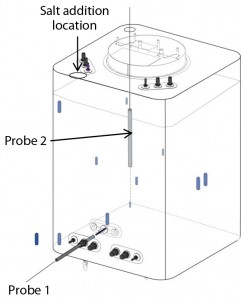
We measured mixing efficiency by adding a 100-mL bolus of sodium chloride (NaCl) solution through a port at the top of a bioreactor containing 200 L of reverse osmosis (RO) water at 37 °C. We determined mixing times for a range of agitation speeds by measuring conductivity over time using two conductivity probes.
One probe was inserted into the standard probe port 1 at the front bottom of the container (Figure 3). Probe 2 was positioned in the back top-left corner, a site shown in preliminary studies with a pH-dependent dye to be the last zone to reach equilibrium. The mixing-time end point was defined as the time required to reach 95% homogeneity (±5% of the final conductivity value). We averaged data from two experiments to obtain a mean value.
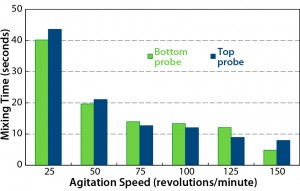
From the results in Figure 4, we can draw the following conclusions: Homogeneity was achieved rapidly (<10 seconds) at the highest speed of 150 rpm. At the lower speed of 80 rpm, which we used for our CHO cell culture studies (2), a mixing time of <15 seconds could be achieved. Both probes recorded similar mixing times, confirming the rapidity of mixing.
Computer Modeling of Mixing Times and Homogeneity: In addition to the experimental work, we developed a computer model of this cuboidal STR bioreactor’s CFD (6). It helped us predict flow patterns and mixing-time responses for a tracer salt solution added to the bioreactor and to compare modeling data with those obtained experimentally.
These computations were carried out for a fully resolved vessel geometry, including baffles and impeller. The model predicted both the velocity profiles and tracer concentrations for simulated addition of 100 mL of a 15% salt solution to a fully developed flow of 200 L of water at 37 °C, agitated at 75 rpm. Then the model computed temporal and spatial changes of tracer concentrations and conductivity values normalized to those achieved after homogeneity. We extracted the time required to reach salt-concentration homogeneity at the locations of the two probes from numerical data and compared that with experimentally derived mixing times.
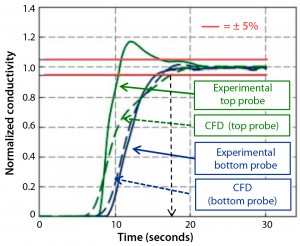
Figure 5: Comparing conductivity signals measured and computed (100 mL of 15% salt solution into 200 L water at 37 °C; agitation 75 rpm)
CFD modeling predicted that conductivity would rise very rapidly in the first 10–15 seconds and then stabilize; that 95% homogeneity would be achieved within 15–18 seconds; and that bioreactor contents would mix without forming dead zones. Those mixing predictions were similar to data obtained experimentally, with <15% difference in mixing times (Figure 5). For all conditions, the absence of curve tails and pronounced signal oscillations support the conclusion that the tracer mixes rapidly within the biocontainer.
Experimentally, an overshoot of 18% in final conductivity could be observed for probe 2, located just below the point of salt addition. This overshoot was always present for low to medium agitation speeds (25–100 rpm) and not for medium to high agitation speeds (data not shown). This transient effect, not present for probe 1, can be explained by the higher-density salt solution gravitating rapidly to the top probe and creating a localized temporary zone of higher salt concentration. With increasing agitation speeds, the concentrated bolus of salt solution is dispersed before reaching probe 2. We did not include this effect in our CFD model, which accounts for the slight difference in the conductivity profiles for probe 2 between the predicted and experimental data. More detailed information on this work can be found in a separate publication from Pall Corporation (7).
Temperature Control: An essential requirement for cell culture performance, precise temperature control is evidenced by a uniform temperature profile throughout the vessel and an accurate and constant temperature set point. Some bioreactors, especially larger-scale systems, can show poor heat transfer and heat loss leading to prolonged heat-up times, temperature gradients, and large temperature fluctuations.
So we studied temperature control in an Allegro STR 200 bioreactor fully filled to 200 L. We placed nine calibrated thermocouples at different locations within the biocontainer and connected them to a Kaye validator (Amphenol Sensors) for data logging. Then we filled the biocontainer with 200 L of chilled reverse-osmosis (RO) water and inserted a Pt100 temperature probe into the sleeve at the bottom of it for direct contact with the fluid. The proportional integrative derivative (PID) controller received a signal from the Pt100 temperature probe. Temperature was cascade controlled, with the fluid temperature as the master control and heater mat temperature as the slave control loop. Agitation speed was set at 150 rpm, airflow at 20 L/min and temperature set point at 37 °C using the controller.
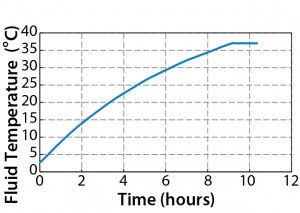
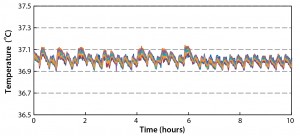
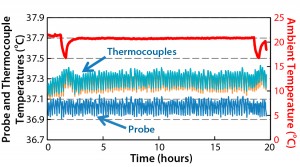
Figure 6 compares fluid temperature against heating time, showing that 200 L of chilled water was heated from 4 °C to 37 °C in 8.9 hours during which the temperature never exceeded the set point. We obtained additional data from the Pt100 temperature probe and the thermocouples after the set-point temperature had reached equilibrium (Figure 7). From these results, we draw the following conclusions: Temperature fluctuations were minimal, with less than 0.1 °C variation around the set point. Temperature values were similar among the thermocouples, within <0.05 °C difference (which can be attributed to equipment and experimental error). We observed no temperature gradient, so temperature was uniform within the biocontainer.
We also investigated temperature stability at the minimum working volume of 60 L with an agitation speed of 50 rpm, an air aeration rate of 6 L/min and a temperature set point of 37 °C. Figure 8 shows a uniform temperature of fluid in the biocontainer and temperatures maintained within ±0.1 °C, with a positive offset of 0.2 °C relative to the 37 °C set point that indicates a greater influence of ambient temperature at this volume. So that offset should be accounted for when working at minimum volumes.
Oxygen Mass Transfer: Because of oxygen’s very low solubility in water at 37 °C, oxygen transfer from air to fluid is a a very challenging requirement when designing and engineering bioreactors. Additionally, mammalian cells have high oxygen demands. To determine the ability of the Allegro STR 200 bioreactor to transfer oxygen, we used a volumetric oxygen mass transfer coefficient (kLa O2) based on the following methodology:
- The gassing out method was used with nitrogen and air through the ring sparger.
- Test fluid was a cell culture media simulant (bicarbonate buffer, Pluronic F68 surfactant, and 50 ppm of Antifoam A) heated to 37 °C.
- Liquid volume was 200 L, and air flow rate was set to 0.1 vvm.
- Data were collected using two electrochemical DO probes (Mettler Toledo) connected to a M800 transmitter and logged by a program written in LabVIEW (National Instruments).
- For 20–80% DO, kLa values were calculated by the following equation, in which DO* refers to DO at saturation, and DO20 and D80 respectively refer to 20% and 80% of DO at saturation:
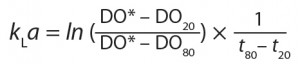
- Probe response time was taken into account for kLa values >70 h–1, using the method described by Sorenson (8).
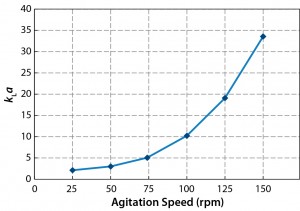
Figure 10 presents the results of our oxygen mass transfer studies. A high kLa value (33 h–1) was obtained at an agitation speed of 150 rev/min; at 80 rpm, which we use for our CHO cell line, a kLa value of 7 h–1 was achieved. So the sparger aeration system and mixing performance were able to fulfill the oxygen requirements for our CHO-S cell culture studies (2).
CO2 Mass Transfer: As a natural byproduct of aerobic respiration in mammalian cell culture, carbon dioxide has the potential to inhibit cell growth, protein production, and product quality attributes such as glycosylation at high CO2 partial pressure (pCO2) levels (9). Although the optimal level of CO2 varies by cell line, enough CO2 to maintain good cell growth usually can be stripped from the fluid surface in small-scale bioreactors through surface aeration and sparged air bubbles. However, the ratio of gas surface area to liquid volume decreases with increasing scale, making CO2 stripping a more difficult challenge for larger-scale bioreactor designs.
Allegro STR 200 bioreactors offer the option of an additional open-pipe sparger to boost CO2 stripping rates, if necessary. So we further studied 200 L of a culture-fluid simulant at 37 °C (as described above) adjusted to pH 6.5 by sparging with CO2 gas, and we maintained that pH for 10 minutes. In a bicarbonate buffer solution, pCO2 established at a specific pH is derived (10) from this equation:
pCO2 = (pHt – pHinitial) × log (pCO2 initial).
After initial pCO2 was recorded with a CO2 probe, we flushed the headspace with air until a DO probe positioned there showed that 95% of the CO2 had been replaced by air. Then we sparged air through the open pipe of the bioreactor at differing flow rates and measured pH until the media simulant had reached pH 7.2. Using equations published by Goudar et al. (11), we derived CO2 concentrations from pCO2 values.
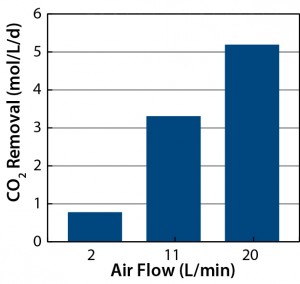
Figure 10 summarizes the CO2 removal data for three flow rates from which we draw the following conclusions: A CO2 removal rate of ≤5 mol/L/d could be achieved at flow rates ≤20 L/min. And at flow rates of 10 L/min (as used in our CHO-S cell culture studies (2), the open-pipe sparging can remove CO2 at least by 3 mol/L/d. These results show that CO2 mass transfer can be managed effectively with single-use technology at a 200-L scale with an Allegro STR 200 bioreactor.
Optimizing Culture Performance
Our studies were designed to help us determine whether a single-use stirred-tank bioreactor could provide control of critical physical parameters that influence mammalian cell culture performance.
By evaluating the hydrodynamics of an Allegro STR 200 single-use bioreactor, we established that the direct-drive large impeller could provide sufficient power input for fluid volumes ≤200 L. In conjunction with a customized cubical biocontainer having integrated baffles, that power transfer produced homogeneous mixing in <10 seconds. Our experimental results on mixing agreed with a theoretical modeling study using CFD. For mass transfer, we confirmed good oxygen transfer rates from the ring sparger that satisfied the requirements of our subsequent CHO-S cell culture studies (2).
In addition, the system achieved effective CO2 stripping through an open-pipe sparger. We obtained precise and homogeneous control across the bioreactor volume: The heater system raised the temperature of 200 L of media from 4 °C to 37 °C in under nine hours. A forthcoming publication will present results of our further studies investigating the cell culture performance of a Pall Allegro STR 200 bioreactor with a CHO-S MAb-producing cell line scaling from 10 L to 200 L.
The transition from cleanable, reusable systems to disposable, single-use technology for complex equipment such as bioreactors is a major challenge for product designers and developers. Our studies have shown that engineering of single-use technology can provide a stirred-tank bioreactor at 200-L scale with fluid dynamics that are suitable for mammalian cell culture.
References
1 Rader RA, Langer ES. Upstream Single-Use Bioprocessing Systems. BioProcess Int. 10(2) 2012: 12–18.
2 Isailovic B, Kradolfer M, Rees B. Performance Qualification of a Single-Use Stirred-Tank Bioreactor with CHO-S Cell Culture. BioProcess Int. 2015: in press.
3 USD 2923: Allegro STR 200 Single-Use Stirred-Tank Bioreactor. Pall Life Sciences: East Hills, NY, March 2014; www.pall.com/ pdfs/Biopharmaceuticals/Allegro_ STR_200_USD2923.pdf.
4 Godoy-Silva R, et al. Physiological Responses of CHO Cells to Repetitive Hydrodynamic Stress. Biotechnol. Bioeng. 103(6) 2009: 1103–1117.
5 Nienow AW. Reactor Engineering in Large-Scale Animal Cell Culture. Cytotechnol. 50, 2006: 9–33.
6 Hoeks FW, et al. Industrial Applications of Mixing and Mass Transfer Studies. Alvin W. Nienow’s Day: Stirred, Not Shaken Symposium, Birmingham, UK, 2004.
7 USD 2980: Characterization and Engineering Performance of the Allegro™ STR 200 Single-Use Stirred Tank Bioreactor System. Pall Life Sciences: East Hills, NY, 2014.
8 Sorensen KL. Thesis Paper 738: Comparative Studies on Oxygen Mass Transfer for the Design and Development of a Single- Use Fermentor. Utah State University: Logan, UT, 2010; http://digitalcommons.usu.edu/ etd/738.
9 Gray DR, et al. CO2 in Large-Scale and High-Density CHO Cell Perfusion Culture. Cytotechnol. 22, 1996: 65–78.
10 Bowers JS. Sparger and Surface Gas Transfer for Cell Culture Bioreactors. American Institute of Chemical Engineers: Philadelphia, PA, 2008.
11 Goudar CT, Piret JM, Konstantinov KB. Estimating Cell Specific Oxygen Uptake and Carbon Dioxide Production Rates for Mammalian Cells in Perfusion Culture. Biotechnol. Progr. 27(5) 2011: 1347–1357. •
Bojan Isailovic is principal R&D engineer, and Byron Rees is senior R&D manager for cell culture applications at Pall Life Sciences, 5 Harbourgate Business Park, Southampton Road, Portsmouth, UK. Corresponding author Michael Kradolfer is global product manager for Pall Life Sciences, Avenue de Tivoli 3, 1700 F Fribourg, Switzerland; 41-26350-5368; michael_kradolfer@europe.pall.com.