The Bolt-on Bioreactor (BoB) project is an independent initiative to develop and commercialize a bioreactor for automated and efficient culture of adherent cells, especially in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. Addressed in the first installment of this series (2), the first challenge concerns volumetric productivity. The second challenge is automating culture processes, the third involves containment and sterility, and the fourth relates to process economics. This four-part series addresses each of those challenges in turn and describes design features that are incorporated into the BoB design for overcoming them.
The second challenge is to develop a system that can automate a continuous culture process for anchorage-dependent cells while preventing cellular stress cycles such as discontinuous medium replacement and intermittent cell detachment. The system should provide homeostatic culture conditions throughout a culture process with programming and manual control options to modify culture conditions. Relevant parameters should be recordable. To address that challenge, we first need to examine the type of process we want to automate: adherent cell culture.
Culture Parameters of Adherent Cells
Initially, a small amount of cells suspended in culture medium is placed on top of a given attachment surface: a continuous flat surface, a roller bottle, or particulated supports. Gentle agitation is used to distribute cells evenly. Normally, attachment surfaces are previously equilibrated with culture medium, and optimum culture conditions are established before cells are seeded.
Next, cells are given some time to settle and attach to their culture surface. When they are properly attached, cell growth and expansion begins. In agitated systems (e.g., roller bottles or stirred tanks), process speed is reached at this point. From now on, process parameters are controlled and actions — such as medium replacement, increasing gas inflow, pH regulation, and thermostatting — are triggered as a response to deviation of process parameters from defined set points. Control of those process parameters may be manual or automated.
For several aspects affecting productivity, continuous culture of adherent cells — e.g., perfusion culture on microcarriers (3) — differs from discontinuous, batch cultures carried out in T flasks or roller bottles. Two differences are stress suffered by cells with every subculture step in batch processes (4) and a reduction of contamination risk in continuous systems (5). Kadouri and Spier highlight several other advantages of continuous culture over batch processes (5). Considering those advantages, the BoB team concluded that extensive use of batch culture systems for adherent cells comes from a lack of suitable continuous culture systems. That makes automation and perfusion key issues in adherent-cell culture design.
Although much of the culture process retains similarities with that of suspension cells, culturing adherent cells involves some particularities arising from the cells’ dependence on available attachment surface to grow in nice monolayers. Some systems such as Synthecon’s Rotary Cell Culture systems provide a means to grow three-dimensional (3D) cultures of adherent cells, but the BoB team is focusing on two- dimensional (2D) cultures. Overall, our process requirements are to provide a gentle and homeostatic system for optimum growth of cells attached to a culture surface. The BoB team aims to develop an efficient perfusion bioreactor for adherent cells.
To achieve that goal, a number of parameters must remain constant: temperature, pH, medium composition (including oxygen and other gases as well as excreted metabolites), surface geometry and composition, agitation rate, and other conditions that might affect culture conditions. We believe that a gentle and homeostatic system for adherent cell culture must control
- culture medium (pH, temperature, composition)
- gaseous phase (temperature, humidity, composition)
- attachment surface (availability, temperature) • other parameters (shear stress, light-derived damages).
Existing culture equipment such as ZellWerk’s ZRP system (and others provided by well-established vendors such as GE Healthcare, Sartorius, EMD Millipore, and Pall Corporation) stabilize those parameters through different methods. For example, providing a constant concentration of CO2 in the gaseous phase works well to stabilize pH to a certain extent when using bicarbonate-based buffers such as Dulbecco’s modified Eagle’s medium (DMEM) or Roswell Park Memorial Institute (RPMI) medium. And changes in pH come from the metabolic activity of growing cells: The more active a cell population is, the faster will changes happen in its medium pH. Therefore, controlling the gaseous-phase composition and/or continuously replacing culture media could be a good strategy for pH control (6). You could also choose to control pH through precise addition of acid or base.
To make things more interesting, bear in mind that some parameters affect others. For example, temperature affects the solubility of gases and thus oxygen availability (7). With an ample choice of strategies, the BoB team wants to ensure that those chosen will best agree with solutions to the other three challenges of the BoB project.
Culture Medium Composition
Cells need nutrients to maintain their metabolic activities. Nutrients are provided by their culture medium, which becomes depleted of nutrients as cells use them up. As it happens, consumption rates differ for each nutrient and change at different stages in a culture process. Glucose is a major example (8). Cells excrete metabolites that affect the culture as well. That effect can be negative (e.g., changes in pH, as above) or positive, such as production of growth factors (9). Dissolved oxygen (DO) is a component of major concern because efficient gas-transfer mechanisms must make sufficient oxygen available to cells (10). A common strategy for dealing with culture medium composition in sophisticated bioreactors is to monitor the concentration of each relevant component (glucose, lactate, ammonia, DO, pH, and so on) and then control its concentration to desired levels through addition of different components. Some perfusion systems (e.g., Novasep’s ATF system) retain suspended cells within a reactor by continuously separating those cells from their culture medium while replacing used media with fresh media.
A strategy followed with traditional adherent cell culture using plates, T flasks, and roller bottles also replaces used medium with fresh medium. In these systems, a certain amount of medium is added to the culture device. The volume added depends on the amount of culture surface area because gas transfer (and therefore oxygen uptake) is affected by the depth of the liquid atop the layer of cells. (This is not the case with roller bottles, however, in which gas transfer occurs when the cell layer is exposed to the gaseous phase as the bottle rotates). The volume of medium added is ~0.5 mL/ cm2, and normally it is replaced every 24–48 hours. The replacement rate depends on pH change, which in turn depends on the cell population’s size and metabolic activity.
But we want to keep cells from suffering stress cycles during medium replacement, so we do not want to wait until pH has dropped to unbearable levels before replacing culture medium. In a 10,000-cm2 (1-m2) culture device (T flask area varies around 50 to 150 cm2, each tray in a CellStack system is about 630 cm2, and a common roller bottle has ~870 cm2), the maximum culture medium replacement rate when confluence is reached will be ~3.5 mL/min (10,000 cm2 × 0.5 mL/day × cm2 ÷ 1,440 min/day). That is easily provided even using very small peristaltic pumps. So a control system that regulates culture medium replacement rate based on the level of a chosen indicator (e.g., pH) will provide a robust method of controlling culture-medium composition. Additionally, some sort of buffering system (e.g., a reservoir of medium within the culture device) can help reduce sudden composition changes as fresh medium is added and used medium is withdrawn from the culture chamber.
In addressing the first challenge (volumetric productivity), the BoB team chose to use a rolled membrane as a cell-attachment surface (2). By contrast with other systems, oxygen uptake in this geometry will occur when cells are exposed to the gaseous phase. Measuring DO in the culture medium thus would be a misleading indicator for oxygen availability. That is not the case when using particulated supports as attachment surfaces (e.g., Cytodex microcarrier-filled culture bags operated in a Wave bioreactor system from GE Healthcare), with which DO is the most important parameter (11). Gaseous-phase composition will be a more robust indicator for oxygen availability in a BoB system. Figure 1 is a schematic representation of the control strategy designed for this new bioreactor.
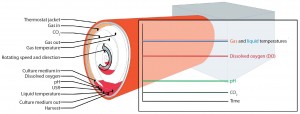
Figure 1: The BoB control strategy; at the onset of a cell-culture process, rotation speed is kept to a minimum to facilitate cell attachment to the culture surface. Once cells have attached themselves, rotation is set to process speed. As the cell population grows, increasing metabolic demand will force a higher culture-medium replacement rate to maintain constant pH. Deviation of CO2 concentration in the gaseous phase and/or dissolved oxygen (DO) in the liquid phase will trigger forced circulation of the gas mixture. Gas flow and the heating jacket will thermostat the system in response to variations from the temperature set point of the liquid and gaseous phases. Excess medium accumulated in the lower part of the culture chamber will act as a buffering system for medium temperature and composition. A user-defined parameter can provide additional process control. The simplified version of the rolled membrane shown here includes only three turns, but many more layers will be found in a real system (2).
Temperature Control
Temperature is very important to adherent cell culture (8). For suspension cells, culture medium temperature alone determines the temperature at which they are cultured. But in adherent-cell culture, cells are exposed to three distinct environments: the solid attachment surface, the liquid culture medium, and the gaseous phase. All three environments (each with its own particular heat transmitting properties) must be kept at the same temperature for optimum culture conditions. The temperature of the cell-growing environment is affected mainly by the temperature of newly introduced culture medium, the temperature of the gaseous phase, and heat transfer from within the culture chamber to the surrounding environment.
In existing culture systems such as T flasks and stacked plates, after a stressing medium-replacement step, culture takes place in a thermostatted environment (an incubator) where cell- attachment surface, culture medium, and gaseous phase get heat from the incubator environment. Roller bottles work in a similar way. As long as the door of the incubator (or thermostatted room) remains closed, this type of thermostatting system works well. With automated bioreactors for suspension cells, it is more common to find heating jackets surrounding the culture chamber. That is advantageous when you want a temperature within the culture chamber that is different from that of the surrounding environment. Using heating sources within a culture chamber is rare because of the extreme temperature gradient that can occur as cells get closer to a heat source (12). Conditioning culture medium and gas before they enter a culture chamber is common to reduce sudden temperature changes with existing systems.
Having analyzed the different options available, the BoB team favors a combination of three elements to control system temperature: a detachable thermostatting jacket surrounding the culture chamber to heat culture medium and prevent heat loss, a culture-medium reservoir within the chamber to act as a heat accumulator, and a heating system for prewarming incoming gas.
pH Control
Metabolic activity of growing cells changes the acidity of their culture medium. Even minute deviations of pH from optimum can be deleterious to growing cells (13). Therefore, a pH-control system must be in place. Some media for cell culture contain buffering systems that successfully regulate pH to a certain extent. CO2 helps maintain the buffering capacity for many such media, whereas others require acid or base addition. Replacement of culture medium as a response to pH changes is a tempting strategy to control culture medium composition and can serve as a pH-control system too. The BoB team plans to control pH through a combination of CO2-containing gas circulation and culture medium replacement.
Gaseous-Phase Composition
Traditionally, adherent cell culture uses air or CO2-enriched air. Oxygen addition is normally unnecessary because metabolic requirements for oxygen usually are satisfied by oxygen contained in the air. However, both CO2 and O2 must be subject to close control, especially for suspension cell culture, because both can seriously affect a culture’s progress. When necessary — for example in use of stirred tanks — oxygen is also introduced into the culture chamber, normally through spargers, but only in high–cell-density cultures because oxygen toxicity can otherwise occur (7). With laboratory devices such as T flasks, culture plates, roller bottles, or stacked plates, gas diffusion between the inside of each device and its incubator through filter membranes is enough. But we are aiming for higher cell densities than traditional laboratory-scale devices allow for adherent cell culture, and metabolic demand of the cultures will in most cases require forced gas circulation.
For temperature control above, we considered conditioning incoming gas before it enters the culture chamber. If more than one gas is to be part of the gaseous phase, you could have each gas enter the chamber separately. However, that would force conditioning each of those gases separately. Therefore, mixing gases before conditioning them is preferable. The gas mixture will be provided directly from an external source; gas temperature, flow, and composition will be controlled by the system. Flow will be kept to the minimum necessary to satisfy metabolic needs. Given the large contact area with the culture-medium– soaked membrane, the gaseous phase will be sufficiently humid within the BoB culture chamber.
Attachment Surface Availability
In plates or roller bottles, adherent cell culture starts with a relatively small cell population that propagates and colonizes all available attachment surfaces. Once confluence is reached, cells are harvested and divided into two or more larger devices to start a new culture cycle: culture passing, passage, or subculture. That process is repeated until the desired number of cells is obtained.
In a BoB system, however, the amount of available culture area within the culture chamber is designed to provide the desired number of cells using a single device. Therefore, once a culture starts and given the continuity of the attachment membrane surface, cells can propagate and colonize the entire surface. That is not possible for systems in which culture surface is not continuous, as with particulated supports. In such systems, cells have a much harder time colonizing particles other than the one they are growing on (14).
Other Parameters
Shear stress is a major concern for culturing cells in stirred tanks (15). Such stress is induced mainly by mechanical agitation of liquid (typically induced by rotary impellers), although air bubbling and foaming also serve as major sources of shear force. Impeller design and antifoaming agents are common strategies to reduce shear stress in suspension cultures.
In small laboratory-scale culture devices, shear forces mainly come from regular medium replacement and subculture cycles, when pipetting and cell detachment/shaking are very aggressive to cells (16). With roller bottles, rotary speed also must be considered because cells require some time to establish a strong surface attachment. Thus, rotary-speed control is important. A balance must be found to ensure that cells are frequently exposed to both their culture medium and the gaseous phase while keeping conditions gentle enough for their attachment, especially during the initial seeding step. Given a sufficiently slow rotation speed, foaming is not a consideration with roller bottles.
For a BoB system, the team finds that rotary speed is an important parameter to be controlled for providing optimum culture conditions. Because cell detachment requires more vigorous agitation, inversion of rotary direction and high speed should be optional at desired points in a culture process. Fluid flow and rotary movement of the rolled membrane also have important effects on medium distribution and mixing.
Whether directly related to certain wavelengths on cells or light-mediated decomposition of culture medium components, deleterious effects of light on cell cultures have been reported (17). Nothing seems to be gained from exposing cells to light, so the BoB system’s thermostatting jacket will protect cultures from light while allowing enough room for visual inspection of the process.
Adherent cell cultures may need control of particular parameters for special strains, culture media, or culture conditions. Additional components may need to be added at particular stages, and pH may need to be controlled through acid or base addition. Therefore, the BoB system must include options for users to expand the number of controlled parameters.
Process Strategy
| These are the major process control strategies devised for culture of adherent cells in a BoB system. You can find a video summarizing these findings online at www. boltonbioreactor.com/about-the-bob-project/the-bolt-on-bioreactor/challenge-2.html. |
| Homogeneous Cell and Medium Distribution: Controlled rotation speed helps distribute cells and culture medium homogeneously across an attachment surface. Reversing direction combined with faster rotation speed will facilitate cell detachment and washing steps. |
| Culture Medium Composition: Medium replacement rate is regulated based on pH variation. Replacement is also used to harvest extracellular products. A culture medium reservoir helps absorb sudden composition and temperature changes in the media. |
| Process Temperature Control: The combined effect of a thermostatting jacket, a culture medium reservoir in the culture chamber, and a heated gas inflow will maintain a constant temperature inside the chamber. Both liquid and gaseous-phase temperatures will be measured continuously. The thermostatting jacket shields growing cells and culture media from the damaging effects of light. |
| Oxygen Availability: A sustained gas inflow and large volumetric mass transfer coefficient (kLa) in the system guarantees that oxygen in the external gas source is made available to attached cells. Flow speed will vary in response to dissolved oxygen (DO) in media and CO2 concentration in the gaseous phase. |
| pH Control: For CO2/bicarbonate buffered media, CO2 contained in the incoming gas flow will help regulate pH. Gas flow will be influenced by CO2 concentration in the gaseous phase. Medium replacement will be the major mechanism for pH control. |
| User-Defined Control: Base addition, additional medium components, specialty gases, and other components can be added based on readings from user-defined parameters. |
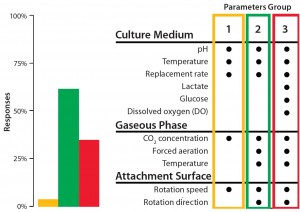
Figure 2: The BoB team has its own views on addressing the four challenges that must be met in designing a future standard solution for adherent-cell culture. Market opinion is most important, however. So as part of its open design initiative, the team is conducting a series of public surveys to provide insight on design features that would best suit market needs. A July 2014 survey asked what process parameters would need controlling to overcome Challenge #2.
An Automation Strategy
We conclude that a reliable automated and continuous culture process for adherent cells requires programmable control of rotation speed and direction; medium replacement rate; thermostatting jacket temperature; incoming gas temperature and flow; CO2 concentration in gaseous phase; liquid-phase temperature and pH; additional, user-defined parameters; and a culture medium reservoir within the culture chamber. However, a significant number of respondents to our survey carried out in summer 2014 considered DO to be a key parameter for controlling in relation to this automation challenge (Figure 2). Therefore, we will include DO in the set of controlled parameters for our new bioreactor. Finally, the “Process Strategy” box describes our control and automation approach.
References
1 Bolt-on Bioreactor: Automated Adherent Cell Culture, 2014; www.boltonbioreactor.com.
2 Simon M. Bioreactor Design for Adherent Cell Culture: The Bolt-on Bioreactor Project, Part 1 — Volumetric Productivity. BioProcess Int. 13(1) 2015: 28–33.
3 Butler M, et al. High Yields from Microcarrier Cultures By Medium Perfusion. J. Cell Sci. 61, 1983: 351–363.
4 CB-0813-03. ATCC Animal Cell Culture Guide: Tips and Techniques for Continuous Cell Lines. American Type Culture Collection: Manassas, VA, 2014.
5 Kadouri A, Spier RE. Some Myths and Messages Concerning the Batch and Continuous Culture of Animal Cells. Cytotechnology 24, 1997: 89–98.
6 Cok JA, Fox MH. Effects of Chronic pH 6.6 on Growth, Intracellular pH, and Response to 42.0 °C Hyperthermia of Chinese Hamster Ovary Cells. Cancer Res. 48, 1988: 2417–2420.
7 Leo P, et al. Animal Cells: Basic Concepts. Animal Cell Technology: From Biopharmaceuticals to Gene Therapy. Castilho L, et al., Eds. Taylor & Francis Group: New York, NY, 2008; 13–38.
8 Zeng A-P, Bi J-X. Cell Culture Kinetics and Modeling. Cell Culture Technology for Pharmaceutical and Cell-Based Therapies. Ozturk S, Hu W-S, Eds. CRC Press: Boca Raton, FL, 2006.
9 Koller MR, et al. Growth Factor Consumption and Production in Perfusion Cultures of Human Bone Marrow Correlate with Specific Cell Production. Exp. Hematol. 23(12) 1995: 1275–1283.
10 Fenge C, Lüllau E. Cell Culture Bioreactors. Cell Culture Technology for Pharmaceutical and Cell-Based Therapies. Ozturk S, Hu W-S, Eds. CRC Press: Boca Raton, FL, 2006.
11 Birch JR, Arathoon R. Suspension Culture of Mammalian Cells. Large-Scale Mammalian Cell Culture Technology. Lubiniecki AS, Ed. Marcel Dekker, Inc.: New York, NY, 1990; 251–270.
12 Henle KJ, Roti JL. Response of Cultured Mammalian Cells to Hyperthermia. Thermal Effects on Cells and Tissues. Urano M, Douple E, Eds. VSP: Utrecht, The Netherlands, 1988; 57–82.
13 McDowell CL, et al. Animal Cell Culture, Physiochemical Effects of pH. Encyclopedia of Cell Technology. Spier RE, Ed. Wiley-Interscience: Hoboken, NJ, 2003.
14 Wang Y, Ouyang F. Bead-to-Bead Transfer of Vero Cells in Microcarrier Culture. Cytotechnol. 31(3) 1999: 221–224.
15 Pellegrini MP, et al. Mechanisms of Cell Proliferation and Cell Death in Animal Cell Culture In Vitro. Animal Cell Technology: from Biopharmaceuticals to Gene Therapy. Castilho L, et al., Eds. Taylor & Francis Group: New York, NY, 2008; 147–180.
16 Xie Y, et al. Pipetting Causes Shear Stress and Elevation of Phosphorylated Stress- Activated Protein Kinase/Jun Kinase in Preimplantation Embryos. Mol. Reprod. Dev. 74(10) 2007: 1287–1294.
17 Mallaney M, et al. Effect of Ambient Light on Monoclonal Antibody Product Quality During Small-Scale Mammalian Cell Culture Process in Clear Glass Bioreactors. Biotechnol. Progr. 30, 2014: 562–570.
Marcos Simón, PhD, is founder of the Bolt- on Bioreactor Project, Technology Park, Mikeletegi 56, 20009 San Sebastian, Spain; marcos.simon@boltonbioreactor.com; www.boltonbioreactor.com.