During the past decade, single-use bioprocessing bags and bioreactors have gained a significant foothold in the biopharmaceutical industry because they offer a number of advantages over traditional stainless steel equipment, especially for clinical production, multiproduct facilities, and emerging economies. At the same time, some companies are concerned that plastic materials might release potentially toxic substances that could affect cell growth and product titers (1). In a worst-case scenario, they could even compromise drug safety when a company uses disposable bags for intermediate or drug substance storage.
Despite the broad acceptance of single-use bags in clinical and commercial biomanufacturing, some researchers have reported recently on inconsistent and poor cell growth of some production cell lines in various single-use bags (2–4). Hammond et al. identified a degradation product derived from a commonly used antioxidant that can be present in some commercially available single-use bags at levels that negatively affect cell growth (5, 6). However, such antioxidants are necessary to keep single-use bags stable: They protect the polymer film during gamma irradiation and protect it from oxidative degradation during extrusion and storage. Hence, it is extremely important to optimize and closely control the concentration of such antioxidants to prevent batch variability.
Other common additives are so called “slip agents,” typically oleamide, erucamide, and stearamide. Such agents act as lubricants and prevent stickiness of polymer film layers, which in turn facilitates bag fabrication and in-process use. Ideally, slip agents should be omitted to further reduce the risk of leaching substances that could potentially affect cell growth.
Innovative Strategy
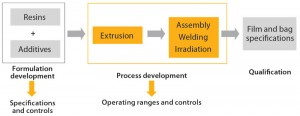
When Sartorius Stedim Biotech began development of its innovative S80 polyethylene (PE) film for the new Flexsafe range of bioprocess bags, the company’s vision was to establish full control and traceability of resins and additives, to fully control the manufacturing process (including film extrusion), and to guarantee industry-leading security of supply to ensure business continuity for the biopharmaceutical industry (Figure 1). The aim was to move away from the current industry practice of using resins or films based on trade names, which in essence provides no true control of polymer and additive formulations. This new film ideally should work for all single-use bag applications, both in upstream and downstream. So it should meet the various physical, chemical, and biological requirements of culture media and chromatography buffer mixing and storage, stirred-tank and rocking-motion bioreactors, intermediate and bulk drug product storage, shipping, and freeze–thaw applications (as described in the “Target Product Profile” box).
Target Product Profile of New S80 Multilayer Polyethylene FilmReproducible cell growth (comparable to that in borosilicate glass)Traceable and controlled polymer and additive formulationRobust and suitable for all single-use bioprocessing bag applications in upstream, downstream, and final filling:
|
To achieve those goals, Sartorius Stedim entered into a strategic partnership with Südpack, Europe’s leading film-extrusion company. Furthermore, we established a strong collaboration with leading polymer and additive manufacturers to access and control the formulation of our polymer and additive package. That is crucial to a strong assurance-of-supply concept.
Development of New Film
Standardized Cell-Growth Assay: During development, 25 different polyethylene multilayer film formulations (based on widely used polymers and European Pharmacopoeia listed antioxidants) were created and evaluated against the defined target product profile (TPP) of the new film. To identify potentially harmful leachables, we established a standardized cell culture assay to examine gamma-irradiated sample bags, as described in the “Standardized Cell Culture Assay” box (3, 4). We understood early on that the typically conducted USP <87> cytotoxicity testing would not be predictive of cell growth behavior.
| Standardized Cell-Growth AssayProtein-free, chemically defined cell culture medium incubated for three days at 37 °C in freshly γ-irradiated sample bags at a volume/surface ratio of 3 cm2/mLControl: medium incubated three days at 37 °C in Duran glass bottle
Method: rCHO DG44 cells grown in six-well plates using the above treated medium |
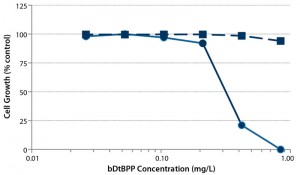
Figure 2: Cytotoxicity of bDtBPP; CHO-DG44 viability (dotted line) and cell growth (solid line) as a function of bDtBPP concentration (7)
No Slip Agents and Optimized Antioxidant Package: Instead of slip agents, we applied nontoxic mechanical antiblocking based on silicon dioxide, which creates a slightly rough surface to counteract stickiness. We optimized the antioxidant package and minimized the concentration of a pharmacopeia-listed, trisarylphosphite-based processing stabilizer (sold under a number of trade names). That component is known to degrade into a reported growth inhibitory substance (5): bis(2,4-di-tert-butylphenyl) phosphate (bDtBPP).
Most important, we established the dose dependency of cell growth and viability for a model recombinant Chinese hamster ovary (CHO) cell line as a function of bDtBPP concentration (Figure 2) to ensure that our cell growth assay would identify nonsuitable formulations, which were evaluated during development of the polyethylene film. Figure 3 illustrates results from some selected film formulations. We found the “S80” formulation to be the most suitable, showing no impact on cell growth in our standardized assay or with other production cell lines (4, film 6).
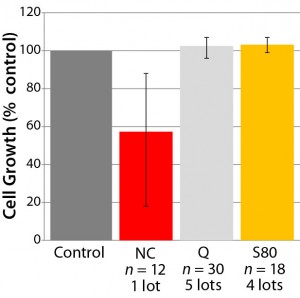
Figure 3: Cell-growth behavior in some selected film formulations; a number of film lots
and sample bags were evaluated. The “error
bar” shows minimum and maximum cell
growth observed.
Extrusion Process Operating Ranges Established
Next, we assessed the impact of extrusion process parameters on cell growth behavior within a defined window. We also performed an expert risk assessment of those parameters and their likely effects on film quality attributes.
The main influencing factor during film extrusion is the amount of heat transferred to process polymer materials. This heat transfer is directly proportional to the oxidation of additives and the quantity of degradation products that are generated to protect the polymer chains. This heat transfer is linked to three critical extrusion process parameters: the melting temperature of resins (extrusion temperature), the cooling temperature of the film (chill roll temperature), and the quantity of material extruded per hour (output).
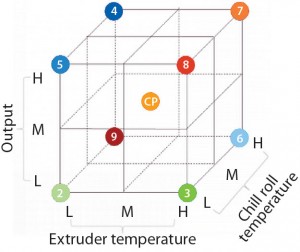
Figure 4: Graphical representation of full
factorial experimental design to establish
operating ranges of critical extrusion process
parameters (extruder temperature, chill roll
temperature, and output); L, M, and H represent
low, middle, and high settings. Center-point
(CP) settings were performed in triplicate. (7)
We used the best film formulation determined in our initial studies and conducted a full multifactorial experimental design (Figure 4). We examined cell growth using our established cell-growth testing approach, demonstrating that the evaluated critical process parameter (CPP) ranges provide consistent cell growth comparable to the control sample results (Figure 5).
No Aging Effects Detected
Because degradation of antioxidants continues during storage of film rolls, after γ-irradiation and dry storage of bags, we investigated the cell-growth performance of our selected film formulation in an accelerated aging study and compared the results with those of a competitor’s bag. Most notably, our S80 film formulation ensures excellent growth behavior right after γ-irradiation until the end of the aging study — representing 36 months (Figure 6) — whereas the competitor’s film results showed an initially poor growth behavior that did recover after six months of dry storage. That suggests further degradation of bDtBPP into nontoxic compounds. So our data support bag reliability from day one until the end of shelf life — batch-to-batch consistency, in other words — whereas other vendor bags may show deviations depending on storage time.
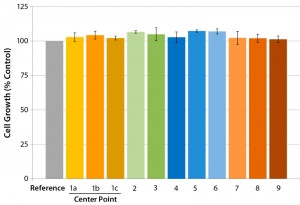
Figure 5: Influence of critical film extrusion process parameters on cell growth examined using the established cell-growth testing approach; error bars represent ±SEM (standard error of mean). The numbers correlate to different experimental conditions of the factorial design; 1a, 1b, and 1c
represent the center-point setting. (7)
No Interaction with Culture Media
This new polyethylene S80 film should work across the full range of bioprocessing applications. Therefore, we were especially interested in understanding cell-growth performance in cold-storage applications with protein-free, chemically defined cell culture media. We considered that to be a worst-case scenario given that serum, hydrolysates, and protein additives can mask detrimental effects of toxic leachates. Gamma-irradiated test bags and borosilicate bottles (control) were filled with chemically defined medium and stored at 2–8 °C for up to six months. No negative effects on cell growth were detected using our cell growth assay (Figure 7) during the storage period.
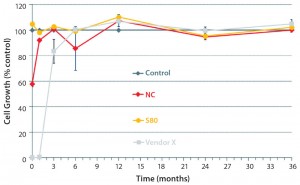
Figure 6: Cell-growth behavior of γ-irradiated bags stored under accelerated aging conditions (36-month representing 337 days at 40 °C); optimized film formulation (S80) showed excellent growth right after γ irradiation until the end of the study (representing 36 months). The negative control (NC) and a bag from another vendor showed initial poor cell growth that recovered after about six months. (7)
Quality by Design Approach to Establish FilmSpecification
Using a quality-by-design (QbD) approach, we established a film specification that ensures excellent and reproducible cell growth, proven by a broad panel of different production cell lines and cell culture media. Together with our strategic collaboration partners in the polymer and film industry, Sartorius Stedim Biotech established full control of the new, state-of-the-art, polyethylene multilayer film formulation as well as traceability and control of polymers and additives used in it.
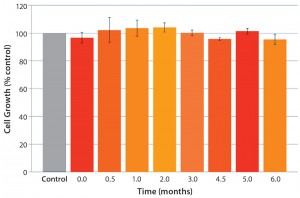
Figure 7: Cold storage (2–8 °C) of chemically defined cell culture medium in S80 polyethylene film
bags; excellent cell growth (comparable to control) was detected over the entire storage period (7).
We applied a multifactorial experimental-design approach to determine operating ranges of the film extrusion. And we established operating ranges for film-welding parameters and γ-irradiation of Flexsafe bags (data not shown). Based on this unprecedented material and biological science and process control approach, we can ensure excellent and reproducible growth behavior for the most sensitive cell lines with Sartorius Stedim Biotech’s Flexsafe bags. This has been confirmed by a recently published interlaboratory test (4), in which a number of bioprocessing bags from different vendors were evaluated using a panel of different production cell lines and media. Other authors have demonstrated the suitability of Flexsafe bags for mesenchymal stem cells (8).
Controlling the complete manufacturing process — from resin to final bag — ensures consistent and reproducible quality for the new generation of Flexsafe bioprocessing bags made with the innovative S80 film. This has been proven by lot-to-lot consistency in cell-growth performance of this new multilayer polyethylene film. Understanding and controlling the film formulation and manufacturing process are key prerequisites to establish meaningful and reliable extractable and leachable data as needed for toxicological assessment of intermediate and bulk drug substance storage and stability studies.
All that combined with a robust assurance of supply concept guaranteeing uninterrupted supply of bioprocessing bags (9) is absolutely crucial to pave the way toward fully single-use bioprocessing facilities of the future. It ensures that a reproducible quality of bioprocessing bags can be supplied throughout the entire life cycle of a biologic drug — from clinical development, when single-use bioreactors and bags might be used for the first time to make or store investigational medicinal products, to many years after market launch of the final drug product.
Conclusion
The material science and process understanding applied during development of the new S80 film — enabled by strategic partnerships in the polymer industry — were cornerstones to delivering the new Flexsafe range of bioprocessing bags. This unprecendented traceability and control aproach answers key needs of the biopharmaceutical industry for single-use factories of the future. Sartorius Stedim Biotech’s new S80 polyethylene film sets the standard with respect to cell growth, robustness, assurance of supply, and batch consistency that are unprecedented in the industry.
Acknowledgments
The authors thank Amgen Inc. (USA) for providing bDtBPP. We also acknowledge Hanni Sun, Oliver Scheibe, and Tanja Frick for excellent technical assistance.
References
1 Wood J, Mahajan E, Shiratori M. Strategy for Selecting Disposable Bags for Cell Culture Media Applications Based on a Root- Cause Investigation. Biotechnol. Progr. 29(6) 2013: 1535–1549.
2 Gammell P, et al. The Impact of Lot-to-Lot Variability of Disposable Cell Culture Bags on Cell Growth During the Scale-Up of a Mammalian Production Cell Line. Cell Culture Engineering XIII, 22–27 April 2012.
3 Steiger N, Eibl R. Interlaboratory Test for Detection of Cytotoxic Leachables Arising from Single-Use Bags. Chem. Ing. Tech. 85(1–2) 2013b: 26–28.
4 Eibl R, et al. Recommendation for Leachable Studies: Standardized Cell Culture Test for the Early Identification of Critical Films for CHO Cell Lines in Chemically Defined Culture Media. Dechema January 2014.
5 Hammond M, et al. Identification of a Leachable Compound Detrimental to Cell Growth in Single-Use Bioprocess Containers. PDA J. Pharm. Sci. Tech. 67(2) 2013: 123–134.
6 Hammond M, et al. A Cytotoxic Leachable Compound from Single-Use Bioprocess Equipment That Causes Poor Cell Growth Performance. Biotechnol. Progr. 22 January 2014 (epub ahead of print).
7 Jurkiewicz E, et al. Verification of a New Biocompatible Single-Use Film Formulation with Optimized Additive Content for Multiple Bioprocess Applications. Biotechnol. Progr. 30 May 2014; http://onlinelibrary.wiley.com/doi/10.1002/btpr.1934/full.
8 Imseng N, et al. Towards a Standardized Biological Test for the Identification of Cell Growth Influencing Leachables (poster). ESACT 2013: Lille, France, 23–26 June 2013.
9 Cappia JM, et al. Enhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with Suppliers. BioProcess Int. 12(8) 2014: S43– S46.
Christel Fenge is vice president of marketing fermentation technologies, Elke Jurkiewicz is a senior scientist in upstream technology R&D, Ute Husemann is manager of upstream technology R&D, Thorsten Adams is product manager of fermentation technologies, Lucy Delaunay is an R&D film and material scientist, Gerhard Greller is director of upstream technology R&D, and Magali Barbaroux is director of film and material R&D at Sartorius Stedim Biotech, August-Spindler- Straße 11, DE-37079 Goettingen, Germany.