 The Xuri cell expansion systems (Xuri W25 and W5) provide a functionally closed, single-use bioreactor that requires fewer operator intervention steps, reduces the risk of contamination, and controls the cellular environment to maximize cell yield. Data presented here highlight that at a cellular level, results from different bioreactors may appear similar, but differences can exist at the receptor expression level.
The Xuri cell expansion systems (Xuri W25 and W5) provide a functionally closed, single-use bioreactor that requires fewer operator intervention steps, reduces the risk of contamination, and controls the cellular environment to maximize cell yield. Data presented here highlight that at a cellular level, results from different bioreactors may appear similar, but differences can exist at the receptor expression level.
Expansion of T Cells
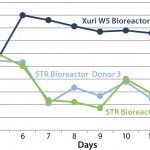
FIGURE 2: An example of how the pO2 saturation can vary during the
expansion of T cells; the lack of effective mixing in the STR bioreactor
reduces the capability of the stirred-tank system to maintain an
acceptable level of dissolved oxygen.
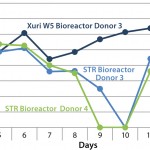
FIGURE 1: An example of how pH can vary during the expansion of T
cells; at higher cell densities, the stirred-tank bioreactors, which lack
perfusion features, are unable to maintain a suitable pH, resulting in the
operator having to exchange medium to correct the pH.
Peripheral blood mononuclear cells (PBMCs) were expanded in static culture. On Day 5, 2-L Xuri Cellbag bioreactors were placed on the Xuri W5 system — at 37 °C, 5% CO2, a 10-rpm rock rate, and 6° rocking angle. Semicontinuous perfusion was initiated when the volume reached 1 L and cell density = 2 × 106 cells/mL.
For the 0.5-L stirred tank (STR) and 1-L gas-permeable static bioreactors, cells were incubated at 37 °C and 5% CO2 until the maximum working volume was achieved. Fresh medium was replenished every 48–72 hours, and cells from the waste medium were returned to the culture.
TABLE 1: Summary of the typical data collected from the expansion of T cells for two donors; the Xuri W5 bioreactor produced higher yield of cells.
Using the % positive as an indicator of phenotype suggests that the systems are similar for CD3/CD4/CD62L or CD3/CD8/CD62L expression.
Phenotypic Analysis
Cellular phenotype was analyzed at day 10 on a BD LSRFortessa™ flow cytometer. Briefly, 1 × 106 cells were labeled with CD3-perCP-Cy5.5, CD4-PE, CD8-Alexa Fluor488, CD28-APC and CD27-V450, or CD57-APC and CD62L-V450.
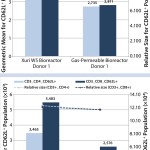
FIGURE 3: How the expression of the key “homing” receptor, CD62L (bars and left-hand y axis), can vary between culturing cells from two donors in the gas-permeable and Xuri W5 bioreactors; cell size is recorded on the right-hand y axis and plotted as a dotted line, illustrating that the difference in object intensity is not a consequence of cell size.
In Summary
Enabling control of the cell culture environment during the expansion of T cells can provides a consistent culture environment with respect to cell health and phenotype. Xuri cell expansion systems offer an automated perfusion approach that can increase cell yield and viability as well as reduce the risks associated with operator intervention.
Ray Ismail (ray.ismail@ge.com) is a senior scientist, Sarah Stone is a stem cell laboratory technician, Bill Shingleton is an R&D team leader, Paul Bowles and Angela Marenghi are development scientists, and Mark Briggs is a senior program manager at GE Healthcare Life Sciences.
Disclaimer: The Xuri cell expansion systems W5 and W25 and Xuri Cellbag bioreactor are not medical devices nor CE marked and should not be used in diagnostic processes. Drug manufacturers and clinicians are responsible for obtaining the appropriate IND/BLA/NDA approvals for clinical applications. GE, Imagination at work, and GE Monogram are trademarks of General Electric Company. © 2014 General Electric Company — all rights reserved. First published October 2013. Xuri is trademark of GE Healthcare Companies. GlutaMax and Alexa fluor are trademarks of Life Technologies. X-VIVO 10 is a trademark of Lonza. LSRFortessa is a trademark of Becton, Dickinson and Company. Vi-CELL is a trademark of Beckman Coulter. Xuri Cell Expansion products are for research use only and not intended for diagnostic use. Drug manufacturers and clinicians are responsible for obtaining the appropriate IND/BLA/NDA approvals for clinical applications. GE Healthcare UK Limited. Amersham Place, Little Chalfont, Buckinghamshire, HP7 9NA UK.
