Lively debate in 2012 concerned the risks and benefits of laboratory studies that created a contagious H5N1 avian pandemic influenza (flu) laboratory-strain virus. One benefit of the public debate is that it reminded governments of the increasingly likely and disastrous possibility of a devastating flu pandemic on the scale of the Spanish influenza outbreak of 1918. Natural evolution of circulating H5N1 viruses could lead to emergence of a deadly and contagious strain (1). Here we outline conventional flu vaccine options and their limitations with regard to pandemic influenza preparedness. We also describe a new approach provided by BiondVax’s M-001 technology for preparedness ahead of pandemic influenza outbreaks.
Background: Countless influenza strains arise as the flu virus mutates unpredictably and frequently, making flu the most common infectious disease. Conventional vaccines rely predominantly on triggering antibody responses to variable regions in the influenza envelope protein hemagglutinin (HA); consequently, they are highly strain specific. Seasonal flu vaccines are manufactured based on global surveillance of circulating strains. Each targets a few influenza strains (three or four) that are considered the most likely to be a health burden in a given hemisphere. Similarly, prepandemic vaccines are manufactured and stockpiled for strains determined to represent the greatest public health threat among potentially pandemic strains causing local outbreaks. So each one targets a specific influenza strain.
The current “reactive” flu vaccine production process often results in seasonal and prepandemic vaccines that target virus strains mismatched to the actual emerging strains. Consequently, conventional flu vaccines can be ineffective (2). Indeed, an accurately matched pandemic vaccine is manufactured only after an outbreak of a pandemic virus begins. So existing influenza vaccine technology results in a time lag during which populations are exposed to a pandemic strain without effective protection (3). Considering the vulnerability of infants and the elderly to seasonal influenza-related complications — and the ever-present public health threat of a deadly influenza pandemic — the world urgently needs a new generation of influenza vaccine technologies that are more broadly protective (4).
To Better ProtectFor the past decade, academic laboratories and companies around the world have been attempting to develop a universal flu vaccine that would protect against all strains for several seasons, the “holy grail” of flu research. Over the years, we have heard both chief executive officers (CEOs) and professors declare that achievement of this goal is just around the corner — “two or five years from now.” However, the reality is that a universal flu vaccine is still several years away. Each universal flu vaccine research program is faced with the time-consuming and expensive challenge of establishing vaccine efficacy while navigating regulatory labyrinths.
Doing so is challenging for two main reasons. First, the classical biomarker used today by regulatory authorities to license conventional flu vaccines is based on detecting antibodies to the virus’s HA-variable region triggered by the vaccine, and they are measured using hemagglutination inhibition (HAI). However, most universal flu vaccine candidates are designed to work differently from how conventional flu vaccines work. So they cannot be measured using the existing HAI immune marker. They would require definition of a new biomarker that better correlates with protection. Second, it is unclear whether a next-generation flu vaccine to replace the standard of care will need to demonstrate superior effectiveness, which could prove economically and practically challenging. Nevertheless, it is reasonable to assume that new biomarkers eventually will be validated and a regulatory pathway established such that a universal flu vaccine can reach the market.
Facing Today’s Pandemic ThreatsSeveral approaches are being taken to incrementally improve conventional strain-specific flu vaccines while allowing them to continue being evaluated using the classical HAI immune biomarker. These are the four main developments:
-
Modifying production such that live viruses are propagated in cell culture; for example, Baxter and Novartis both have succeeded in registering seasonal flu vaccines manufactured using Vero cells (Preflucel) and MDCK cells (Optaflu), respectively.
-
Modifying production such that recombinant HA proteins are made in cell culture, as accomplished by Protein Sciences Corporation and registered in the United States
-
Adding extra virus subtypes into vaccines (5), as exemplified by the quadrivalent seasonal products (to be available next season) that will contain two A- and two B-type flu strains (6).
-
Adding adjuvants — such as Fluad MF-59 (Novartis) and Pandemrix AS03 (GlaxoSmithKline) — which can somewhat broaden the cross-reactivity of vaccines to nonconstituent flu strains and are licensed for flu vaccines only in Europe.
The first two approaches address the speed of manufacturing, likely reducing it from six to eight months down to about four months. Obviously, the speed with which a government can obtain a flu vaccine targeted against an emerging pandemic strain is likely to affect the spread of disease as well as associated morbidity and mortality, so those approaches do address the challenge of pandemic influenza risk management to some extent. The latter two steps address the breadth of strain coverage, so to some extent they could also contribute to pandemic influenza risk management.
In the relatively conservative flu vaccine field, those incremental steps actually represent huge leaps involving significant financial investment. Nevertheless, there are still no flu vaccines that can be manufactured ahead of pandemic outbreaks and thus be ready for delivery to the population at the very moment such an outbreak occurs.
The “Universal” AlternativeOur company is taking an alternative (not mutually exclusive) approach to improving pandemic preparedness and enhancing the effectiveness of conventional flu vaccines. BiondVax is a clinical-stage biotech company dedicated to improving global protection against flu. The main focus is development of a universal influenza vaccine candidate called M-001, which was pioneered in the laboratory of world-renowned immunologist Ruth Arnon at the Weizmann Institute in Israel.
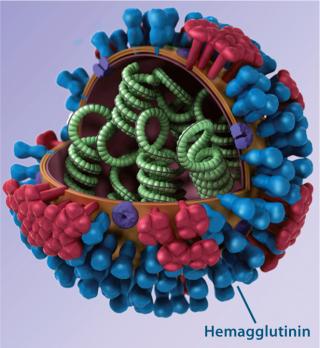
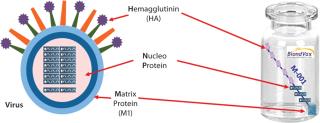
An epitope-based recombinant vaccine, M-001 triggers both cellular and antibody immune responses to parts of the virus that are conserved and common among influenza A and B seasonal and pandemic strains. Specifically, this proprietary recombinant protein is manufactured in bacterial cells and comprises a unique combination of nine select, conserved viral regions (peptides) taken from HA, nucleoprotein (NP), and M1 matrix proteins of influenza (Figure 1). Because those regions are common deno
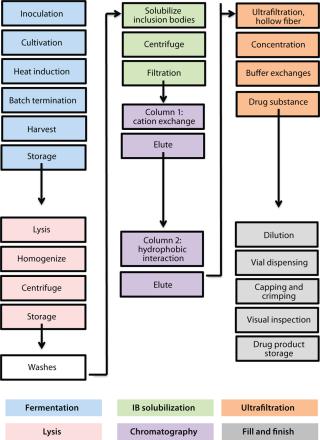
minators for all flu strains, M-001 represents a universal vaccine designed to protect against all strains, present and future. The multiple-strain antiinfluenza activity and safety of this vaccine have been demonstrated through several animal studies and four clinical trials (two phase 1–2 and two phase 2) involving 440 adults (age 18–65) and elderly volunteers (>65 years old) (7). The composition of M-001 is unchanging, which enables the vaccine to be manufactured and administered year-round — independent of surveillance and season. The robust fermentation, purification, and formulation process takes only six to eight weeks (Figure 2).
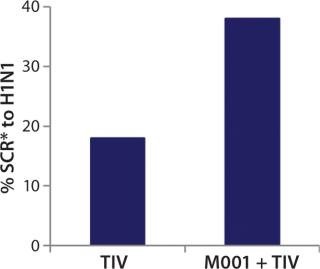
While investigating the vaccine’s activity, BiondVax discovered that when it is given as a primer a few weeks before a boost of conventional flu vaccine, M-001 increased the proportion of participants responding sufficiently to the three strains contained within the seasonal flu vaccine (as measured by the HAI biomarker). Not only did this prime-boost regimen potentiate the immunity triggered by a conventional flu vaccine, but it also allowed for measuring M-001’s efficacy indirectly (after the boost) using the classic biomarker. When administered in a prime-boost regimen, M-001 has improved immune responses to every flu strain in each boost tested to date, supporting the premise that it represents a “universal” influenza vaccine. Specifically, priming with M-001 three weeks before the 2011–2012 seasonal flu vaccine resulted in significantly more elderly persons (aged >65) seroconverted towards the constituent H1N1 pandemic swine influenza strain (A/California/7/09) than did administering the seasonal vaccine alone (Figure 3).
Those findings represent the first-in-human demonstration of a universal influenza vaccine priming a pandemic influenza strain without addition of adjuvant to the prime or boost vaccine. In light of those data, BiondVax predicted that M-001 priming of pandemic influenza vaccines (e.g., the avian pandemic H5N1 vaccine) could improve the required pandemic vaccine dose schedule (enabling one rather than two doses). Indeed, the company found that priming mice with M-001 before administering H5N1 pandemic vaccine led to all the mice exhibiting immunity to the virus such that just one H5N1 vaccine dose (instead of two) was required. The mouse study provides proof of concept for the immunological and dose-sparing benefits of M-001 priming for a pandemic avian influenza vaccine. The proposed mechanism of action for that synergy is amplification of the T-cell response (and ultimately the HAI antibody response) due to overlapping antigenic epitopes between M-001 and the conventional influenza vaccine (or live virus).
For Future Pandemic Preparedness: Designed as a universal flu vaccine candidate, this product can be included in a prime-boost regimen to improve immune responses to all conventional flu vaccines. The resulting synergy endows M-001 with a capability to transform pandemic influenza preparedness, as envisioned in 2012 by Robin Robinson, director of the Office of Biomedical Advanced Research and Development Authority (BARDA) at the US Department of Health and Human Services (HHS) (8).
We propose following a pandemic preparedness plan (PPP) whereby the M-001 universal influenza vaccine is stockpiled during interpandemic phases to enable immediate response to pandemic outbreaks. When an outbreak occurs, the stockpiled vaccine would be administered immediately to the population while a conventional, strain-specific pandemic vaccine is characterized and manufactured (which takes up to six months). M-001 priming would help governments ensure that fewer people suffer from pandemic illness by heightening antiinfluenza immune responses (improving response rates to a conventional pandemic vaccine) and extend the coverage of pandemic vaccines (because one pandemic vaccine dose per person would be required instead of two).
Our prime-boost approach to pandemic preparedness has one distinctive advantage over all other approaches: It eliminates the need to predict the next pandemic strain. M-001 can be administered as a universal pandemic primer come-what-may, which affords governments a time window in which the exact nature of a pandemic outbreak can be characterized and a strain-specific vaccine can be manufactured. So unlike the 2009 H1N1 pandemic situation, when the prepandemic stockpile was a mismatched H5N1 vaccine — or today, when the emerging potentially pandemic strain is H7N9 and once again the stockpile is a mismatched H5N1 vaccine — we propose a new paradigm in which the stockpiled vaccine M-001 is a universal pandemic primer to match all potential pandemic flu strains. As stated in the 2009 H1N1 influenza improvement plan, “Because pandemic influenza represents not just a health threat, but also a threat to all aspects of our society, a comprehensive whole-of-community approach to preparedness remains an important component of successful pandemic preparation” (9). Our prime-boost regimen would enable an immediate, whole-of-community action that could be executed upon the outbreak of a pandemic to prepare the population psychologically as well as immunologically.
But M-001 is not just a primer; it is by design a universal influenza vaccine. In mouse studies, we have found that administration of it before exposing the subjects to a live virus provides greater virus immunity than does exposure to live virus alone (as assessed using HAI biomarker). So we anticipate that after administration of M-001 and before immunization with a pandemic vaccine, M-001 will reduce the burden of pandemic illness.
Finally, because M-001 is manufactured in a fully scalable, robust fermentation process, low-,
moderate-, and high-income countries should be able to maintain national stockpiles. Manufacturing capacity would be easily modulated according to demand. Thus, the prime-boost regimen addresses both the recommendation calling for a recombinant influenza vaccine initiative (10) and the third goal of BARDA’s strategic plan for 2011–2016 “optimizing the BARDA emergency manufacturing response” (11).
Given the potential pandemic nature of an emerging deadly avian H7N9 strain in China (12), we consider the capability of M-001 to transform pandemic preparedness to be of high importance and relevance to global public health. BiondVax is working to ensure that this innovation is licensed in time to make a difference.
Author Details
Tanya Gottlieb is head of business development, Dr. Shimon Hassin is chief operating officer, and Dr. Tamar Ben-Yedidia is chief scientific officer at BiondVax Pharmaceuticals Ltd., Science Park, 14 Einstein Street, PO Box 4143, Ness Ziona, 74140, Israel; 972-8-9302529, fax 972-8-9302531;