CRM197 is a nontoxic mutant of diphtheria toxin containing a single amino-acid substitution of glutamic acid for glycine. CRM197 is a well-characterized carrier protein used in a number of approved and highly successful conjugate vaccines including Pfizer’s Prevnar® pneumococcal vaccine, a multibillion-dollar franchise; HibTiter® (Haemophilus influenzae b) vaccine, and Novartis’ Menveo® meningococcal vaccine.
Traditionally, CRM197 is produced by fermentation of a Corynebacterium diphtheriae C7 (β197) tox (–) strain, which secretes it into the culture supernatant. It is then recovered after separation of biomass and purified using filtration and chromatographic steps. Typically, titers and overall yields are relatively low.
Pseudomonas fluorescens is a useful host for high-level production of recombinant proteins. Pfenex Inc. has developed a BSL-1 P. fluorescens production strain that produces high levels of CRM197, along with corresponding purification methods. Both cGMP and preclinical grades of Pfenex-produced CRM197 are commercially available. The preclinical grade material is produced using an identical process and production strain as are used for manufacturing cGMP CRM197. Use of preclinical CRM197 for early stage vaccine development assures continuity of supply for stage-appropriate grade and quantities of CRM197 minimizing change when a candidate vaccine development program advances through clinical and commercial stages.
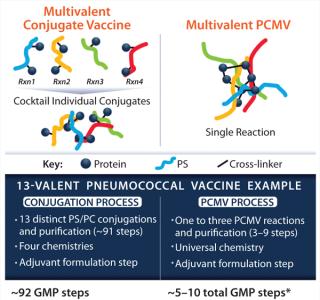
OverviewAs presented by Kevin P Killeen, PhD (chief scientific officer at Matrivax) a proprietary Protein Capsular Matrix Vaccine (PCMV) platform technology, or “virtual conjugate,” entraps polysaccharides in a cross-linked CRM197 protein matrix. This eliminates the need to create covalent bonds for connecting each polysaccharide to a carrier protein, enabling multivalent polysaccharide entrapment, which streamlines the manufacturing process (Figure 1). Sourcing the preclinical Pfenex-produced CRM197, Matrivax rapidly completed development of a Vi-antigen–based, enteric fever (Typhoid) PCMV candidate. Based on generated preclinical data, Matrivax has seamlessly transitioned toward GMP production using cGMP-grade Pfenex CRM197. Using high-quality, readily available products such as Pfenex’s CRM197 can rapidly progress vaccine development.
Currently, Pfenex CRM197 is being used by 17 different companies worldwide for their conjugate and virtual conjugate vaccine programs, with product candidates at various stages of clinical development. Both cGMP and preclinical grades of CRM197 produced in P. fluorescens are commercially available through Pfenex’s Reagent Proteins division. The ability to rapidly translate novel vaccine-based product concepts into the clinic through such relationships and business models will help to strengthen the overall vaccine research industry by allowing ideas to be materialized and validated in shorter time spans.
Author Details
Cassidy Brady is senior marketing manager at Pfenex Inc., 10790 Roselle Street, San Diego, CA 92121; 1-858-344-7207;