With a compound annual growth rate potential of ∼52% during 2010–2015 (1), the global biosimilars market represents a significant driver in biologics development and manufacture. Increased competition, quality-by-design (QbD) directives, and rising costs are compelling biosimilars manufacturers to search for advanced technologies they can use in optimizing production processes to remain competitive and maximize new opportunities. Here, we discuss biomanufacturers’ needs for robust, standardized cell-culturing procedures that comply with QbD directives. We also describe an effective new NMR-based bioanalysis technology.
Photo 1:
PRODUCT FOCUS: BIOSIMILARS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: ANALYSTS, PROCESS ENGINEERS
KEYWORDS: CELL CULTURE DEVELOPMENT, MEDIA, QBD, BIOSIMILARS
LEVEL: INTRODUCTION
Biosimilars and the Biologics Market
According to Global Biosimilars Market Forecast to 2015, the biosimilars industry will continue to grow throughout the next few years, and patent expirations will provide huge potential to new market entrants (1). A Global Industry Analysts survey predicts protein drug sales will exceed US$158 billion (€122.6 bn) by 2015, with therapeutic antibodies emerging as the market leader (2). First-generation biosimilars dominate the current market across both regulated and unregulated markets, including the United States, Europe, India, China, and Korea. Second-generation products are expected to have significant impact on future markets (1).
In addition to improved safety and efficacy, drug companies need stronger patent positions to protect their investment and secure long-term revenues. More resilient to economic influences than other small-molecule drugs, biologics are already playing a major role in healthcare markets. Globally it is predicted that biologics will make up around 17% of the market by 2016, with 7 of the top 10 global medicines being biologic by then (3). As a result, the development and access to technologies that will address the technical challenges associated with biologics development and production (including new biological entities, biosimilars, and “generics”) will become more of a focus among manufacturers. Such solutions will be the key condition to commercial success (2). NMR for Cell Culture Media Analysis
The biopharmaceutical industry relies heavily on cell culture media in cell line development. Typically, cell cultures are developed for consistent and high-product yields by making use of media with optimum composition that are defined mostly by using an empirical trial-and-error approach. However, that approach can be inefficient and expensive, especially because current bioreactors have limits on their miniaturization. Modern demands call for a better-targeted selection and better analysis and monitoring during process development.
By gaining an in-depth understanding of cell culture characteristics, manufacturers of high-value therapeutic proteins can further implement QbD into bioprocesses and reduce both cost and time of set-up for large-scale cell cultivation. They can also identify and remove cell culture batches that do not comply with specifications, thereby preventing variability further downstream. A good understanding of the factors limiting cell growth and the parameters that can affect protein production can improve therapeutic yields. More important, it can help companies limit batch-to-batch variations and significantly reduce manufacturing failures.
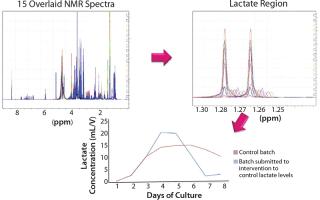
Although liquid chromatography may be the traditional approach for quantitative analysis of feed components and metabolites, as a technique it suffers from throughput and limitations in the number and type of components that can be analyzed at any one time. Spedia-NMR (nuclear magnetic resonance) analysis from Spinnovation Biologics represents the next generation in bioprocessing analytical technology. It uses NMR spectroscopy to evaluate biological samples, cell culture media, and raw materials, offering significant advantages over other techniques. This technology can profile complex matrices and has been shown to predict the performance of cell media in a manufacturing setting. A nondestructive technique with simple sample preparation, NMR is fast, highly reproducible, and capable of measuring more than 60 components simultaneously (Figure 1). Case studies show how this technology is better suited to the current need for process optimization within biologics manufacturing.
NMR Technology Applied to Bioanalysis
NMR is a well established technique for elucidating chemical structure and solving analytical problems. Proton (hydrogen-1) NMR spectroscopy measures changes in the magnetization orientation and magnitude of a hydrogen-1 spin nuclei excited by radio-frequency within a strong magnetic field. The parameter detected is the nutation frequency of the spin nuclei. Data obtained are directly related to the chemical structure, absolute quantity, and dynamics of an observed molecule. NMR measurements can therefore nondestructively identify and quantify components in native and spent media, intracellular media, and even overall organic materials contained within downstream solutions.
In practice, hydrogen-1 NMR is very robust and flexible analytical tool for the biologics industry. New-generation high magnetic fields ranging from 500 to 900 MHz and cryocooled probe technology provide access to increased sensitivity and resolving capabilities, as well as improved automation. NMR can therefore make an extended contribution to current good manufacturing practices (CGMPs), QbD, and process analytical technology (PAT) in biologics manufacturing. Benefits of NMR Optimization
NMR delivers both qualitative and quantitative data that can support the rapid detection and identification of unknown components in nondefined plant- or animal-sourced media. It is sensitive to pH and the presence of trace elements. And it can measure more than 60 feed components, contaminants, and metabolites from a single cell culture medium simultaneously.
Spinnovation Biologics’ NMR methods deliver rapid throughput and advanced data treatment, including pattern interpretation and statistical analysis. Cell culture media studies using NMR techniques can provide a profile over a determined time period, thereby showing the interrelationship between consumption of feed components, release of metabolites, and eventual accumulation of toxic compounds. Multicomponent analysis can be carried out regardless of whether samples
are polar or nonpolar, volatile or nonvolatile. Gathering accurate component concentration data of batch samples is straightforward and rapid, enabling manufacturers to follow the progress of a biologics process. Limits of detection of ∼1 µM can be expected.
When producing biological drug candidates, manufacturers should rapidly understand and monitor the factors that are most influential to cell-growth and production. Among those factors, feeding strategy and the accumulation of potentially toxic metabolites play a central role. To address such issues, Spedia-NMR technology provides rapid and reliable profiling of culture media to support cell culture development and upstream processing.
Our technology can support QbD directives by characterizing nonchemically defined media and other raw materials for bioproduction, link NMR profiles to performance for predictive assays, and identify factors influencing cell culture performance. As a result, biologics manufacturers can troubleshoot and predict performance of nonchemically defined media batches before starting production processes. Media Profiling
Spun out from methods initially developed to analyze bodily fluids, the Spedia-NMR profiling approach can automatically derive the concentration of tens of analytes present in culture media and spent media. Those include all amino acids, more than 10 saccharides, tricarboxylic acid (TCA) cycle components, and many other metabolites and surfactants.
Typologies and intensities of NMR peaks are specific to each analyte and its concentration. In this way, a quantitative profile of a medium can be produced and the consumption and production of components monitored during cell cultivation. The method is applicable to cellular systems such as Chinese hamster ovary (CHO), stem cells, insects cells, bacteria, yeasts, and algae. The highly reproducible results are linear over a broad concentration range (100 µM – 1 M) and do not require systematic calibration. Performance Prediction
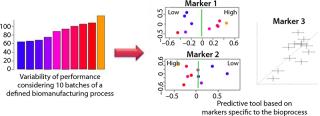
NMR-based Spedia-Predict technology correlates NMR profiles with cell culture performance parameters to define the most influential markers and develop models to predict future performance (Figure 2). It is applied to troubleshoot performance variations in bioprocesses that are related to raw materials and culture media batches. As a result, our technology helps to reduce performance variability and prevents failure of large-scale cell culture.
Spedia-Predict technology allows manufacturers to detect and identify markers that correlate to process performance (e.g., biomass or protein expression). For cell media manufacturing, it improves quality control. The use of such fast and predictive tools addresses issues relating to timelines and costs in large-scale biomanufacturing. An Effective Process Tool
The current market focus on biosimilars will drive future biologics manufacturing and will require manufacturers to become ever more prone to look toward innovative technologies that would enable time and cost savings. Traditional cell culture development methods, relying on trial-and-error selection for raw materials and feed strategy, can no longer be the only approach to upstream processing. More methods enabling better control and understanding should be brought into the picture.
New and innovative NMR-based bioanalysis technologies (tailored specifically to the needs of bioprocessing and manufacturing of biologics) can be used over a range of different production platforms and cell systems to provide insight into culture processes and reduce failure and variability in manufacturing. Process optimization will ultimately save resources and costs, delivering the advantages that biologics manufacturers need in an increasingly competitive marketplace.
Author Details
FC Girard is CEO at Spinnovation Analytical BV, Toernooiveld 1 Mercator III, 6525ED Nijmegen, The Netherlands; F.Girard@spinnovation-analytical.com.
REFERENCES
1.) Global Biosimilars Market Forecast to 2015. ASDReports: Amsterdam, The Netherlands, March 2012.
2 Global Industry Analysts, Biologics Market Worth $158bn by 2015, New Report; in-Pharmatechnologist.com; 19 August 2010.
3 The Global Use of Medicines: Outlook Through 2016. IMS Institute for Healthcare Informatics, July 2012; www.imshealth.com/deployedfiles/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/Global%20Use%20of%20Meds%202011/Medicines_Outlook_Through_2016_Report.pdf .