Taking full advantage of high cell-specific productivity of recombinant proteins requires use of robust fed-batch processes involving addition of one or more concentrated nutrient solutions. Proportionately large amounts of concentrated nutrient supplements are often needed to support the stoichiometrically balanced metabolic requirements of high-density cell cultures. Large feed additions ultimately dilute the final product at harvest, but attempts to minimize feed volumes often lead to multiple concentrated acidic and basic subgroups. Those impart additional complexity to manufacturing processes.
When optimizing basal media and feeds, increasing key component concentrations (fortification) can help increase product titers. However, alternative methods must ensure maintenance of specific productivity later in culture. Considering the difficulties intrinsic to providing desired concentrations of key nutrients in fed-batch culture, an ideal additive would be highly concentrated, single-part, pH-neutral, and stable in both liquid and dry formats. It also could be applied to existing feeds as a partial replacement for reduction of existing volumes and would not affect final product quality.
Using a novel technology, we developed an additive that meets those criteria and overcomes many nutrient supply limitations. Here we outline how this new, functional additive has proven to be highly effective with several Chinese hamster ovary (CHO) cell lines producing immunoglobulin G (IgG) antibodies. Even without process optimization, relatively low proportional additions have resulted in over twofold improvement in product titers of several CHO fed-batch processes. The resulting ability to develop highly concentrated, pH-neutral solutions that can be delivered to any CHO culture system represents a fundamental transformation of fed-batch process development.
PRODUCT FOCUS: ANTIBODIES
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: PROCESS AND CELL CULTURE ENGINEERS
KEYWORDS: CULTURE ADDITIVES, SCALE-DOWN, SCALE-UP, PROCESS OPTIMIZATION, HIGH DENSITY
LEVEL: INTERMEDIATE
Background
Traditionally, process development scientists have added concentrated nutrient supplements to prevent depletion of important nutritional compounds when batch CHO cell cultures experience recombinant protein production limitations (1, 2). Fed-batch processes must be robust to maximize final product concentration through leveraging high viable-cell density (VCD) and cell-specific productivity (qP) (3). To support the stoichiometrically balanced metabolic requirements of high-density cell cultures and satisfy rising nutritional demands at larger production scales, such strategies have naturally led to increased volumes of supplement addition. Proportionately large feed additions can dilute recombinant products at harvest and present liquid preparation, handling, storage, and/or shipping complications during bioprocess scale-up and manufacturing (4, 5). Therefore, it is desirable to minimize liquid volumes and concentrate components as much as possible. However, pH-neutral nutrient solutions cannot be substantially concentrated without adversely affecting component solubility and stability.
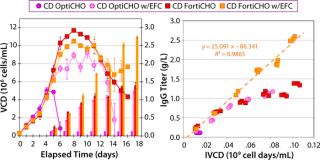
Getting around those difficulties traditionally involves separating components into multiple concentrated subgroups with different high and low pH values (6). However, that method can produce nutrient solutions with short shelf lives. It can also complicate pH control strategies for bioreactors while substantially increasing culture osmolality levels. Efforts have been made to fortify relevant component concentrations of integrated basal media and feeds to reduce the total volume of addition and minimize product dilution (2, 7). In our hands, such strategies have provided benefits we associate with the enriched environment (Figure 1). However, not all cell lines may be successful under such augmented conditions. In addition, process development organizations can become “locked in” to existing fed-batch processes because of limited resources or regulatory considerations, which makes it difficult to consider substantial process changes.
Noting the difficulties in providing desired concentrations of critical components, we applied novel technology to develop a new way to combine key nutrients in a highly concentrated (150 g/L) pH-neutral additive called FunctionMAX TiterEnhancer, which is designed to reinforce existing feeds and amplify the productivity of standard high–cell-density fed-batch platforms. This technology could transform recombinant drug bioprocessing by raising component concentrations to levels that are out of reach using conventional approaches. We have confirmed that dry and liquid formats of the additive are stable for over a year.
We demonstrated 35–120% product titer improvements when adding the FunctionMAX TiterEnhancer product on top of existing processes or as a partial feed replacement for a lower total proportion of feed addition. Such increases were achieved primarily by maintaining qP throughout each fed-batch process, and improvements came immediately without any process optimization effort. We found that low-volume addition with FunctionMAX TiterEnhancer additive was sufficient to improve titers for several processes using different commercially available media and feeds — and those with higher cell densities saw the most benefit. We also demonstrated that productivity can be made comparable to levels attained with enriched media and feeds but without altering product quality.
Materials and Methods
Unless otherwise indicated, all media and reagents were supplied by Life Technologies. Data that were not produced from experiments using internal cell lines (Case Studies 1 and 2) were generated by collaborators in case studies using their own proprietary cell lines.
Case Study 1 (CS1): Proprietary IgG-expressing CHO cells maintained in CD CHO medium were inoculated into shake flasks at 50-mL volumes. Control cultures were fed with 10% CHO CD EfficientFeed B (EFB) feeding supplement on days 4, 6, 8, and 10 (40% total). The FunctionMAX TiterEnhancer product was added to parallel cultures at 3.5% on days 4, 6, 8, and 10 (14% total). As required, gluco
se was added to maintain a concentration greater than 2 g/L in culture. Starting on day 3, cell counts were measured daily using an Innovatis CEDEX cell counter (from Roche Applied Science). And starting on day 6, antibody titers were measured daily with a protein A HPLC system.
Case Study 2 (CS2): Proprietary IgG-expressing CHO cells maintained in CD CHO medium were inoculated into 5-L bioreactor cultures. The control culture was fed with 5.6% EFB on days 3, 5, 7, 9, 11, 13, and 15 (40% total). The FunctionMAX TiterEnhancer product was added in parallel at 1.4% on days 3, 5, 7, 9, 11, 13, and 15 (10% total). Glucose was added as required to maintain its concentration at >2 g/L in culture. Starting on day 3, cells were counted daily as above. Antibody titers were measured daily starting on day 3 as above as well.
Multiple Media Experiment 1 (MME1): We directly inoculated an IgG-expressing internal CHO DG44 cell line (maintained in CD OptiCHO medium with 6 mM l-glutamine) into multiple vendor media with 6 mM l-glutamine. Cell cultures were maintained in all media for seven passages before being inoculated at 65-mL shake-flask cultures containing 4 mM l-glutamine. We fed CD OptiCHO cultures with 10% CHO CD EfficientFeed A (EFA) feeding supplement on days 4, 6, and 8 (30% total), and we fed vendor media cultures according to recommended protocols. The FunctionMAX TiterEnhancer product was also added at 3.3% on days 4, 6, and 8 (10% total) where indicated. We supplemented all cultures with a 30% w/v glucose solution as required to maintain the glucose concentration above 3 g/L. Starting on day 3, we quantified cells using a Vi-CELL XR cell counter (Beckman Coulter) every two days. Starting on day 5, we measured antibody titers using a ForteBio Octet QK system (Pall Life Sciences) every two days.
Multiple Media Experiment 2 (MME2): We also maintained the same CHO DG44 cell line in CD OptiCHO medium with 6 mM l-glutamine and then directly inoculated those cells into CD FortiCHO medium with 6 mM l-glutamine. Cell cultures were maintained in both media for seven passages before being inoculated into 60-mL shake-flask cultures. We fed some CD OptiCHO cultures with 10% EFA on days 2, 4, 6, and 8 (40% total); others were fed with 5.3% EFA on days 4, 6, and 8 (16% total). We added FunctionMAX TiterEnhancer product at 5.0% on day 0 and 3.0% on days 4, 6, and 8 (14% total). CD FortiCHO cultures were fed with 10% CD EfficientFeed C (EFC) feeding supplement on days 4, 6, and 8 (30% total). We supplemented all cultures with a 30% w/v glucose solution as required to maintain the glucose concentrations above 3 g/L. Cell counts and antibody titers were measured as described for MME1.
Glycan Analysis of MME2: We purified IgG from media using POROS protein A MabCapture resin (Life Technologies) and then prepared it as described by Laroy et al. (8). N-linked glycans were enzymatically removed using PNGase F, then purified by graphite carbon SPE. We labeled the glycans with 8-aminopyreme-1,3,6-trisulfonic acid (ATPS) using standard reductive amination (DMSO, 15% acetic acid, and 1M sodium cyanoborohydride). Then we purified those labeled glycans using Bio-Gel P2 size-exclusion chromatography (Bio-Rad Laboratories) and analyzed them using a 3500 Genetic Analyzer instrument (Life Technologies). We identified glycans according to retention time relative to the GeneScan 600 LIZ size standard ladder (Life Technologies), then quantified them using 100-pmol maltohexose and/or maltopentose internal standards labeled with ATPS.
Results and Discussion
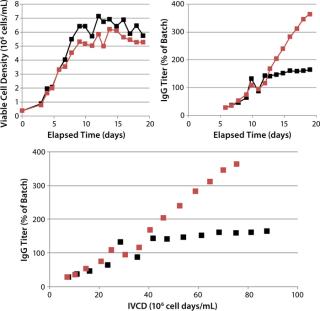
In CS1, an increase of 120% over the control IgG titer was achieved (~3 g/L) by including FunctionMAX TiterEnhancer additive in the existing EFB process. Little change was observed in cell density profile, so the titer improvement can be attributed entirely to qP. The cell-specific rate of production improved 10-fold over the control condition after days 11 and 12 (Figure 2). Neither lactate nor ammonia was significantly different from control results.
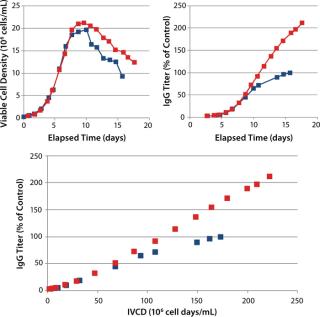
Although a minimal amount of FunctionMAX TiterEnhancer product was added on top of the control CS1 process, it was thought that the total volume of addition could have been maintained if it had been added as a partial feed replacement instead. This was tested with a different cell line in CS2 in 5-L bioreactors, which produced a similar effect. An increase of 110% over the control IgG titer was realized, again demonstrating extension of the initial culture qP (Figure 3). The cell density profile was slightly higher than in the control condition, so in this case not all the productivity enhancement can be attributed to the increased qP.
In those case studies, osmolality never extended beyond ~350 mOSm/kg, and it actually decreased over time. In theory, this would allow for osmolality to be used as a variable for process optimization rather than a constant that needs to be managed. Other case studies were performed (data not shown) that showed similar titer improvements. In one case, a 15% addition gave an increase of nearly 150% over the control IgG, and in another just a 4% addition gave an increase of 50% over the control condition.
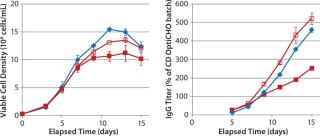
In MME1 (Figure 4), the volumetric productivity of higher–cell-density cultures exhibited a proportionately more positive response to the addition of FunctionMAX titer enhancer. Addition to EFA in CD OptiCHO medium gave 100% IgG titer improvement; addition to Vendor Feed 1 (VF1) in Vendor Medium 1 (VM1) gave 70% titer improvement; and addition to VF1 in Vendor Medium 2 (VM2) gave 35% titer improvement. It appears that starting with a more robust and productive baseline process can lead to greater proportional improvement.
For instance, in the VM2 example
(with cell density ≤5.0 × 106 viable cells/mL) it is reasonable to believe that the cells did not sufficiently challenge the nutritional depth of that medium. Consequently, nutritional limitations present in the culture would be minor, so including the FunctionMAX TiterEnhancer additive had a reduced benefit. It would follow that the response would be most positive if cell culture growth were so vigorous that even a well-designed fed-batch profile could not keep pace with the cells’ nutritional demands.
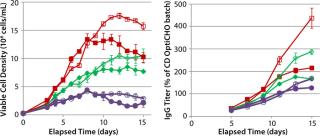
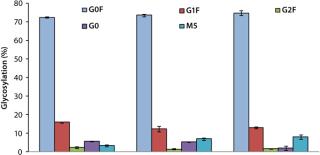
In MME2 (Figure 5), even a partial replacement of EFA with FunctionMAX TiterEnhancer additive and reduction in the total volume of addition (from 40% down to 30%) could deliver 105% IgG titer improvement. This experimental condition also gave 15% better productivity relative to CD FortiCHO and EFC. So the use of fortified media and feeds is not an absolute requirement for achieving high product titers. Consequently, major process changes are not a prerequisite for process improvement. Furthermore, we detected no significant glycosylation differences in the final IgG harvest from CHO DG44 fed-batch cultures with or without addition of the FunctionMAX TiterEnhancer product (Figure 6). Those results indicate that use of the additive should not adversely affect product quality.
Low-volume FunctionMAX TiterEnhancer addition has improved product titers substantially for several CHO fed-batch processes. It can be included in any cell culture system as an add-on to an existing fed-batch process or as a partial feed replacement resulting in the total addition volume being either sustained or reduced without impeding titer increase. We have confirmed that FunctionMAX TiterEnhancer addition can help maintain qP across the entire duration of fed-batch cultures. Our approach has been particularly effective in improving volumetric productivity for processes that achieve higher cell density.
We also demonstrated that FunctionMAX TiterEnhancer addition can raise performance to levels comparable to cultures that feature enriched media and feeds. Furthermore, no effect was seen in measured aspects of product quality. The new additive possesses attributes that could be considered ideal in a functional additive for reinforcing specific productivity in high-density fed-batch processes. The next step in development of this novel technology will produce complete pH-neutral feeds that are highly concentrated and stable. The technology also can be applied in customized solutions to transform process feed formulations into a highly concentrated, single-part, pH-neutral format.
FunctionMAX, EfficientFeed, OptiCHO, and FortiCHO are trademarks of Life Technologes; POROS, MabCapture, and LIZ are registered trademarks of Life Technologes; Bio-Gel is a registered trademark of Bio-Rad Laboratories; Vi-CELL is a registered trademark of Beckman Coulter; Octet is a registered trademark of Pall Life Sciences.
Author Details
Corresponding author Shawn L. Barrett, Ryan Boniface, Prasad Dhulipala, Mark Stramaglia, and Stephen F. Gorfien are in the Bioproduction Division of Life Technologies, 3175 Staley Road, Grand Island, NY 14072; 1-716-774-0249; shawn. shawn.barrett@lifetech.com. Peter Slade and Yolanda Tennico are staff scientists with the Molecular Probes division of Life Technologies, 29851 Willow Creek Road, Eugene, OR 97402; 1-541-465-8300. Peggy Lio is a senior process science fellow with Life Technologies, 7305 Executive Way, Frederick, MD; 1-240-379-7754.
REFERENCES