Influenza vaccine has historically been produced in embryonated chicken eggs. However, to meet the needs for pandemic preparedness and the scalability of vaccine production, cell-based processes are now being developed and implemented in the industry. The methods for purification processes have typically involved a combination of sucrose density gradient ultracentrifugation, ultrafiltration/diafiltration (UF/DF) with hollow-fiber membranes, and chromatography using affinity, ion-exchange, and/or gel filtration media (resins). In these processes, both sucrose density gradient ultracentrifugation and gel filtration have limitations in scalability and throughput. Clearly, new techniques are needed to improve productivity and economy in vaccine production.
Capto™ Core 700 is a member of GE Healthcare Life Sciences’ Capto line of chromatography media (1). The Capto Core 700 medium has a core bead design and consists of an inactive outer layer and a ligand-activated core. Small contaminant molecules enter into the beads where they are captured. Viruses and other large entities with a molecular mass (Mr) greater than approximately 700 000 (700 kDa) are excluded and are collected in the flowthrough. The octylamine ligands in the core of the bead are multimodal, being both hydrophobic and positively charged. These ligands bind various contaminants strongly over a wide range of pH and salt concentrations.
This study evaluates Capto Core 700 as an alternative to current chromatography technologies used in vaccine processes. An experiment to determine the window of operations for influenza virus purification was performed using PreDictorâ„¢ 96-well plates for high-throughput process development. In addition, the impact of Capto Core 700 on process economy was compared to that of Sepharoseâ„¢ 4 Fast Flow, a gel filtration medium often used in the vaccine manufacturing industry.
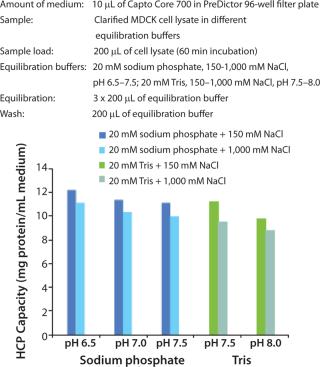
Protein Binding Capacity and the Operating Window
The robust performance of the multimodal octylamine ligand in a relatively wide range of NaCl concentrations and pH (Figure 1) gives Capto Core 700 a wide window of operation. This reduces the need for optimization such as buffer exchange or dilution between steps (even with different feed materials).
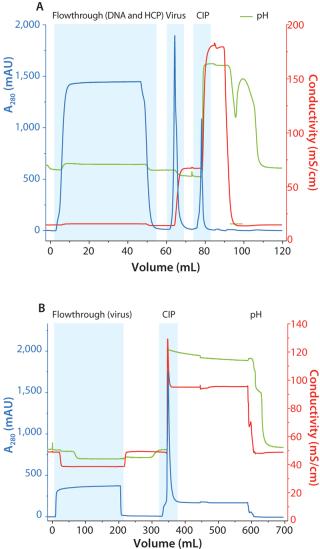
Microfiltration and Two-Step Chromatography Using Capto DeVirS and Capto Core 700
A two-step chromatography process was performed using Capto DeVirS for capture and Capto Core 700 for final viral purification. Figure 2 shows chromatograms from both steps following microfiltration. Capto DeVirS was effective for the capture of the virus (Figure 2A) and eluted fractions from this step were applied to the XK column packed with Capto Core 700 for final purification (Figure 2B). On account of the robust binding performance of Capto Core 700, equilibration of Capto Core 700 was achieved using the buffer used for elution in the Capto DeVirS step. The need for buffer exchange or dilution between steps was thereby eliminated, contributing to a relatively rapid chromatography process. This demonstrates one of the advantages from the wide window of operation that is enabled by Capto Core 700.
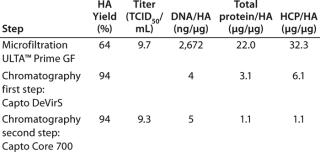
Table 1 shows the results in terms of hemagglutinin (HA) recovery, TCID50, DNA, and protein removal at each step of the process. In this case, good yield of virus HA as well as significant removal of HCP and DNA were observed. DNA was reduced 2.8 log and proteins five- to seven-fold by Capto DeVirS. Capto Core 700 further reduced protein levels by three- to five-fold. The infectivity of the virus was retained throughout the process, as shown by the titer measured with TCID50. (Further examples of purification with Capto Core 700 can be found in reference 2).
Cost Analysis of Capto Core 700 versus gel filtration
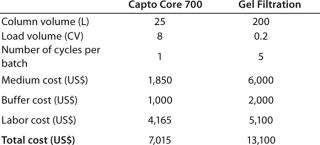
Based on the running conditions and results for the Capto Core 700 example in Table 1, and from experience of using gel filtration, a cost comparison for processing a feed from a 1000 Lfermentation is presented in Table 2. The higher load volumes achieved with Capto Core 700, relative to gel filtration, resulted in smaller volumes of chromatography medium to process the same amount of material within a shorter time-frame. As a consequence, labor costs, buffer costs, and costs for Capto Core 700 medium were significantly lower than for traditional gel filtration. The resulting total cost per batch for this step could be lowered by almost two-fold.
Table 1: Virus hemagglutinin (HA) yield, TCID50, DNA, total protein, and host cell protein (HCP)/HA quotients in a purification scheme incorporating microfiltration and two-step chromatography using Capto DeVirS and Capto Core 700
Table 2: Key process parameters and cost comparison per batch for p
rocessing a 1,000 L fermentation volume; one approach uses Capto Core 700 and the other uses traditional gel filtration (for further details on these cost comparisons, see reference 2)
Conclusion
Capto Core 700 allows for a wide window of operation for pH and NaCl concentration, securing robust purification and simplified process design in vaccine manufacturing. The combination of microfiltration, capture of virus with Capto DeVirS, and final purification on Capto Core 700 showed excellent results in terms of virus purity and reduction of DNA and HCP. Using Capto Core 700 in this approach has the advantage of allowing direct transfer of eluted fractions containing target virus without the need for buffer exchange or dilution, resulting in faster and simpler processing. Cost analyses show that the higher loading capacity of Capto Core 700 leads to shorter process times using less medium, and thus significant cost reductions relative to traditional gel filtration procedures.
Author Details
Anna Åkerblom is a research engineer, and Peder Bergvall is a scientist at GE Healthcare Life Sciences, Björkgatan 30, 751 84 Uppsala, Sweden; www.gelifesciences.com/bioprocess. GE, GE monogram, imagination at work are trademarks of General Electric Company. Capto, PreDictor, Sepharose, and ULTA are trademarks of GE Healthcare companies.
REFERENCES