Clinical cellular therapy applications often times require a cell expansion or maturation step before use. Traditionally, cell expansion or cell culture is performed in “open” systems including multiwell culture dishes or tissue culture flasks. These “open” steps present risks and are not ideal for larger-scale manufacturing. The new EXP-Pak™ cell expansion container is a closed-system, gas permeable bag intended for expansion and culture of nonadherent cells.
EXP-Pak™ bags are made from a unique polyolefin film that permits gas permeability required for cell expansion and maintenance of cell viability in addition to excellent clarity (when filled with liquid) for viewing of cultures. The bags also feature a tubing harness that allows for filling, sampling, and manipulation steps to take place in a completely closed, sterile manner.
The aim of this study was to investigate the ability to culture and expand cells in the EXP-Pak™ (Charter Medical, Ltd. Winston-Salem, NC) and to also compare expansion rates and cell recovery to alternatively available cell expansion bags (different manufacturers). The results herein demonstrate that the EXP-Pak™ can effectively promote cell culture and expansion.
Methods
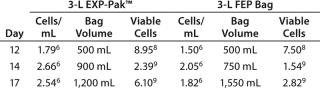
Tumor Infiltrating Lymphocytes (TILs): Briefly, TIL cells for the REP (rapid expansion protocol) were thawed and placed into culture plates. On day 3, culture flasks were set up to initiate the expansion using ≥ 1.0 × 106 cells in 150-mL media. On day 10 of the expansion, ≥1.5 × 108 total viable cells on average were transferred in 500 mL media to either the EXP-Pak™ (3L) expansion bag or previously validated FEP (3L) bag for final cell expansion and comparison. On days 12, 14, and 17, cell counts and viability were performed and cell culture media was added accordingly.
Human Th2 Rapa Cells: Briefly, cultures are initiated with CD4+ selected lymphocytes and placed into culture in X-Vivo 20 media (Lonza) containing 5% HIautologous or HI AB plasma, 20 IU/mL IL-2 (Chiron), 1,000 IU/mL IL-4 (Cell Genix), and 1 µM rapamycin (Sirolimus) in either the EXP-Pak™ bag or the PL732 Lifecell® (Baxter) bag. Cells are stimulated on Day 0 with 3:1 (beads per cell) anti-CD3 and anti-CD28 coated beads (4.5 µM Dynal tosylactivated). For both 6 day and 12 day, 10% 10× (IL-2 and IL-4) media containing 5% plasma and 1 µM rapa is added on days 2 and 4.
Data Summary
Expansion of TILs was performed using the EXP-Pak™ cell expansion bags and compared with a previously validated FEP bag (different manufacturer). Overall cell expansion (cells/mL) and total viable cells in the EXP-Pak™ increased at each of the time points tested in the study (Table 1). Furthermore, when compared to the currently accepted FEP bag used, cell/mL and total viable cells in the EXP-Pak were equivalent or better at each of the days measured. Data from three EXP-Pak™ bags were averaged and compared to the average of six FEP bags. This study demonstrates that effective TILcell expansion can be performed using the new EXP-Pak™ bags.
Table 1: Tumor infiltrating lymphocyte expansion
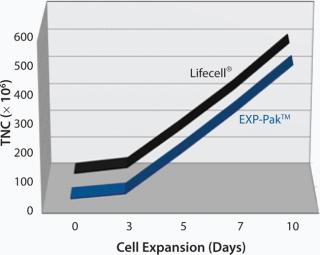
In a separate study, EXP-Pak™ bags were investigated for the expansion of Th2 lymphocytes and compared to cell expansion values achieved using the Lifecell® culture bag (Figures 1 and 2). In the initial experiment, Th2 Rapa cells were cultured and expanded for 10 days in both bags. TNC counts were performed on days 3, 5, 7, and 10 as shown in Figure 1. Similar expansion rates were noted for both bags.
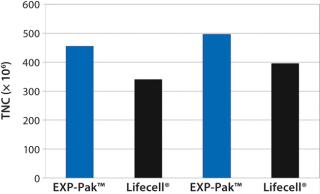
In the second experiment, Th2 Rapa cells from two donors were expanded in EXP-Pak™ bags and compared with Lifecell® containers (Figure 2). Following six days of cell expansion, TNC counts were performed for each donor pair. Results demonstrate similar to slightly improved overall expansion using the EXP-Pak™ bags for both comparisons. Results indicate that EXP-Pak™ bags can be used for Th2 lymphocyte expansion. While additional studies are warranted, the data indicate that comparable values to the Lifecell® container can be achieved with the new EXP-Pak™ closed-system expansion bags.
Conclusion
The data demonstrate that the EXP-Pak™ cell expansion bags provide an ideal environment for culture and expansion of cells. Average cell expansion values (TNC) and total cell viability were demonstrated to be comparable to alternative bags used. Furthermore, the bags provide a completely closed system for sterile filling, sampling, and cell manipulation. In conclusion, the EXP-Pak™ cell expansion containers from Charter Medical, Ltd. offer an optimal, scalable cell expansion environment for a variety of therapeutically important cell types.
Author Details
Dominic Clarke, PhD, is cellular t
herapy and cryogenic storage product manager at Charter Medical, 3948-A Westpoint Boulevard, Winston-Salem, NC 27103; dclarke@lydall.com; www.chartermedical.com TIL data performed and provided by Sabine Ellwanger, MLS (ASCP), cell therapy technologist, and Renee Smilee, MT (ASCP), manager of the cell therapies facility at Moffitt Cancer Center (Tampa, FL). Th2 Rapa data provided by Vicki Fellowes, NIH Department of Transfusion Medicine, Charley Carter Cell Processing Laboratory. EXP-Pak™ is a trademark of Charter Medical, Ltd. (Winston Salem, NC); Lifecell® is a registered trademark of Baxter Healthcare (Deerfield, IL).