Living systems rely on enzymes to perform many essential functions for survival. One prime example is digestion, the conversion of food into energy. Each enzyme possesses specific requirements for the types of molecules that it can use as substrates or reactants to convert to products. Here, I provide some basic information about enzymes, explain their biochemical parameters (e.g., kinetic parameters) and significance for characterization, and review related assays currently available to the bioprocess industry.
Lactose intolerance is a common enzyme deficiency throughout the world. It describes the inability or insufficient ability to digest lactose, a sugar found in milk and milk products. It is caused by a deficiency of the enzyme lactase, which is produced by cells lining the small intestine. Lactase breaks down lactose into two simpler forms of sugar — glucose and galactose — which are then absorbed into the bloodstream.
PRODUCT FOCUS: ENZYMES, VACCINES
PROCESS FOCUS: FORMULATION, PROCESSING
WHO SHOULD READ: FORMULATORS, PROCESS DEVELOPMENT
KEYWORDS: CHARACTERIZATION, PROTEINS, KINETICS
LEVEL: BASIC
By some estimates, 75% of people today are gradually losing their ability to produce lactase. Besides abstinence from dairy products, the palliative treatment is oral introduction of lactase enzyme. For enzymatic products, regulators and reviewers are requiring methods to provide verification of activity as well as purity and identity.
Enzymes are mostly proteins that act on a substrate to provide a product through catalyzation. There are many different types of enzymes. Some enzymes require metal ions, and others require cofactors for maximal efficiency. Enzymatic efficiency is affected by temperature, pH, and the presence of similarly sized and shaped molecules called inhibitors. A partial list includes oxidases, catalases, reductases, proteases, nucleases, gyrases, and isomerases. Lactase is a hydrolase because it separates a molecule after addition of water.
An enzyme functions as a catalyst in a chemical reaction. Catalysts increase reaction rates by lowering the activation energy for product formation. Activation energy is the minimum amount of energy required for molecules to react together in a chemical reaction. The black line in Figure 1 shows the energy pathway for a reaction in the presence of an enzyme. The difference in energy between the crests of the blue and black lines indicates the benefit of an enzyme to conversion of substrate to product. Because of the spatial arrangement of functional groups on the active site, an enzyme can stabilize the transition state of a reaction and therefore reduce activation energy, but not the overall energy of a reaction.
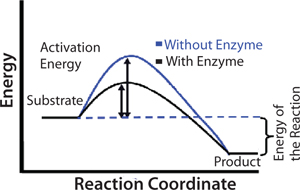
Figure 1:
Therapeutic Use: Lactase uses two critically positioned carboxylic acid groups to stabilize the transition state and speed scission of the bond between glucose and galactose. Researchers have used their understanding of this mechanism to aid treatment of some enzymatic disorders. So the treatment for lactose intolerance is oral administration of lactase pills to supplement any existing levels.
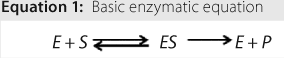
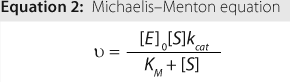
To be used as a therapeutic agent, an enzyme must be characterized — specifically by testing it for purity and identity. In addition, it must be characterized for its activity (how well and efficiently the enzyme converts substrate to product) and kinetic parameters (intrinsic parameters that are specific to the enzyme-substrate set). Kinetic constants confer limits on an enzyme as to its binding capacity, maximum velocity, and turnover rate.
Reactions: Understanding activation energy helps develop a chemical reaction. Equation 1 shows a basic enzymatic reaction, in which S is a substrate, E is an enzyme, and P is a product. The complex formed by an enzyme and substrate is indicated as ES, which is formed in steady-state conditions. An enzymatic reaction such as this is the proposed route for lactase-induced hydrolysis of lactose. The enzyme is present on both sides of the chemical reaction because it acts as a biochemical catalyst.
The principle of steady-state reactions is widely used for dynamic molecules such as enzymes. Most enzymes in living cells reach a steady state. Equilibrium represents death. Steady state is a term used frequently when describing enzymatic processes. It is the situation in which the rate of formation is balanced with the rate of destruction. At the beginning of the 20th century, Michaelis and Menten developed theories to explain this process. They discovered that the velocity of a reaction is generally proportional to enzyme concentration. However, velocity usually follows saturation kinetics with respect to the concentration of a substrate.
The blue line in Figure 2 represents the changing velocity of a reaction with respect to substrate concentration [S]. At low concentrations, velocity increases linearly. At higher [S], linearity of the velocity deviates significantly from linear, and the velocity tends to slow. At very high [S], the reaction rate approaches a maximum termed the Vmax.
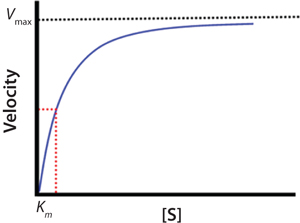
Figure 2:
The relationship between velocity and substrate concentration is described mathematically by the Michaelis-Menten equation (Equation 2), in which ? is velocity, [E]0 is the initial enzyme concentration, [S] is the substrate concentration, kcat is the maximum turnover rate, and KM is a constant related to apparent binding affinity of enzyme to substrate or (as indicated by the red-dotted
line) equal to the concentration at one-half Vmax.
In reality, there are many types of enzyme reactions related to substrates:
-
zero-order, in which rate is independent of substrate concentration
-
first-order, in which rate is directly proportional to substrate concentration
-
second-order, in which rate is proportional to the square or to first power of each of two different substrates.
The mathematics around higher-order enzymatic reactions increases in complexity. Other types of kinetics are double displacement or “Ping-Pong” kinetics, in which a transient covalent complex forms in an active ingredient and two are products produced (1).
Experimentally, you can determine KM and Vmax by plotting your data according to accepted linear plots. When data from a Michaelis–Menten plot is transformed into linear plots, kinetic parameters can be determined. A Lineweaver–Burk plot graphically displays the relationship between velocity and substrate concentration as a double-reciprocal plot. From such treatment, the y-intercept provides Vmax as 1/Vmax and the x-intercept provides the apparent binding affinity as 1/KM. Many researchers today avoid data treatment because it weights the data at higher concentrations more heavily in the analysis.
As an alternative, an Eadie–Hofstee plot does not compress higher values and is considered superior. Kinetic parameters are found in the slope of the line (KM) and the y-intercept (Vmax). A variation of the Eadie-Hofstee is the Scatchard plot, in which data are plotted as the reciprocal of the Eadie–Hofstee plot. The ratio of V/[S] to V is defined as the Scatchard plot. Although such data treatments and plots will continue to be used, software packages such as GraphPad (GraphPad Software, Inc.) and SigmaPlot (Systat Software, Inc.) also can provide parameters through nonlinear regression.
Analytics for Determining Activity and Kinetic ParametersEnzymatic activity measures an enzyme’s catalytic ability. Assays to measure activity use conditions relevant to linear region of the curve in Figure 2. By contrast, kinetic parameters are the intrinsic characteristics of an enzyme with respect to KM, Vmax, and kcat. Calculations of those parameters are derived from nonlinear regression analysis or mathematical treatments of the data.
Analytical techniques for determining activity and kinetic parameters for an enzyme and substrate include
-
direct spectrophotometry (UV)
-
direct spectrofluorimetry (fluorescence)
-
automated spectrophotometric and spectrofluorimetric procedures
-
enzyme coupled assays
-
automatic titration of acid and base
-
radioactive procedures.
For GMP-compliant projects, a spectrophotometer and other equipment must be validated in the laboratory where such work will be carried out. Instruments with software that complies with 21 CFR Part 11 make some US Pharmacopeia (USP)–required baseline corrections impossible. Therefore, wells containing initial solution are alternatively defined as “blank” reactions. An instrument’s software can mathematically base subsequent readings on that initial reading.
Activity: Compendia-based procedures (USP, Europe Pharma-copoeia, Japan Pharmacopoeia) describe many enzymatic assays that use direct spectrophotometric analysis of activity. Although these methods are validated for use, some feasibility studies are usually required to be performed to harmonize a method with existing laboratory equipment. Typically, method feasibility focuses primarily on instrument suitability and preparation of a sample for an assay (e.g., correct dilution and evaluation of matrix for inhibiting compounds).
A suitable spectrophotometric starting point should be provided or determined. Spectrophotometers and plate readers for activity assays include Agilent 8453 UV spectrophotometers and Molecular Devices M2e or M5e MultiMode microwell plate-readers with SoftMax Pro 5.4 or Thermo Scientific’s Varioskan Flash plate readers with dispensing units. The dispenser adds “stop” reagents for quenching reactions at predesignated times. Nonlinear regression analysis using third-party software (e.g., GraphPad Software) helps determine KM and Vmax.
Lactase (β-galactosidase galactohydyrolase) hydrolyzes glycosidic bonds specifically at galactose. USP defines a unit of lactase activity as: “One USP Lactase Unit is the lactase activity contained in the amount of enzyme that hydrolyses one microequivalent of galactosidic linkage per minute at a pH of 4.5 and at 37 °C” (2). The substrate is the synthetic ortho-nitrophenyl-d-galacto-pyranoside.
A general procedure for determing lactase activity of a sample involves transfer of substrate solution to three test tubes labeled S, U, and B. Place the tubes in a thermostated water bath at 37.0±0.1 °C and allow them to equilibrate to temperature. Following incubation, add substrate to tube S, a USP-based standard solution to tube U, and water to tube B (reagent blank). Mix each tube thoroughly mixed and immediately return it to the 37.0±0.1 °C water bath. After a preset incubation time, raise the pH by adding a basic solution such as dilute sodium carbonate and further dilute to procedural guidelines with a volume of water. Read the absorbances of the resulting three solutions with a spectrophotometer at a unique wavelength (specific for the ortho-nitrophenyl group). The USP monograph for lactase provides an equation to relate the observed absorbances to activity as defined by USP Lactase Units. Potency or activity is reported in USP units per gram of sample.
Newer methods replace some USP monographs and rely on synthetically prepared substrates. For example, commercial kits for determining amylase, protease, and nuclease activities respectively use a synthetic lipid (triacylglycerols), fluoroscein-labeled casein, or an oligonucleotide with fluorescently labeled appendages. Those conjugates release a fluorescent dye in real time when a target substrate is cleaved. Such assays are common for research and are gaining creditability with analytical laboratoriess and government agencies.
Kinetic Parameter Determinations: Saturation must be observed to accurately assess Vmax and KM. Although saturation may not be possible for all enzymes (e.g., catalase with hydrogen peroxide substrate due to high velocity of the reaction), it is definitely preferred, and the initial goal of any method development project.
Data are generated using an automated system approach to generate more data points. Generally, a microwell-plate format is preferred and should be evaluated early in development. Automation can measure UV or fluorescence signal at regular intervals in real time, generating >100 data points with 30-second intervals. To determine kinetic parameter, a nonlinear curve analysis as fitting the Michaelis-Menten equation follows. Overall, if more data points are included in the curves, then the values derived are more accurate.
Other methods for enzyme characterization have found specific applications in the industry. Titration with acid and base is an assay for which the detector is a glass pH electrode. Reaction volumes can be small and limit of detections can be in the nanomolar range. However, atmospheric CO2 may influence the readings if prolonged exposure occurs.
Enzyme-coupled assays rely on secondary a
nd some tertiary reactions to provide a signal. To make such assays possible, it is critical to select a secondary enzyme with a much greater kcat (turnover rate) and Vmax than the enzyme that is being characterized. That is necessary so as not to have a “pooling” of a product from the target enzyme. Subsequently, the secondary enzyme system provides a substrate for a third enzyme system. An example is an enzyme-assisted redox reaction such as the conversion of NADH to NAD+. That conversion is observed spectrophotometerically by monitoring 340 nm. However, many variables should be taken into account when planning those types of experiments. First, the activity of the second and third enzymes must be confirmed and provided from a reputable and reliable source. Also, the redox reaction uses a substrate that may be air sensitive. If so, then the automated reading may be contrary to expectations because of the reaction of atmospheric oxygen.
Radioactive methods have been relied upon for decades and are highly sensitive. Various isotopes used include 3H, 14C, 32P, and 35S to achieve attomole detection levels (from 10–18 to 10–17 moles). Some drawbacks to this procedure are low-efficiency readings (aqueous solutions), quenching, and unwanted tritium transfer. Finally, with methods using radioactive isotopes, a laboratory facility requires licensure for possession and storage of such materials. A current trend has been to develop fluorescence-based assays with comparable sensitivities.
Understanding Basic PrinciplesCharacterizing enzymes as therapeutics requires careful consideration about their clinical phase development. In addition to testing for purity and identity, it is important to assess the activity and kinetic parameters of a therapeutic enzyme. Therefore, a good understanding of these basic principles can provide a basis to devise assays for complete product characterization. In general, most stability programs underscore the importance of activity measurements. However, many fail to understand that modification of a single residue (amide-to-carboxylic acid by means of deamidation) can severely alter the binding capacity of a substrate. That can affect KM, so the kinetic parameter characterization may be stability indicating.
With the wide range of techniques possible to help analytical scientists today — and the advent of newer techniques — determination of those parameters should be straightforward. For development projects, all resources should be considered, including vendor kits, synthetic substrates, and the sometimes outdated compendia procedures.
Modernization of assays is of critical concern to regulators. Legacy methods were recently discussed at the 15th Annual Well-Characterized Biopharmaceuticals (WCBP) in Washington DC, January 2011. Such assays were developed and validated in the past and, because of increasing technology, are being challenged by newer, more-sensitive techniques. As a result, an increasing number of methods are being revalidated with new sets of specifications. The conclusion of this forum was that comparability must be demonstrated between new and old methods.
Author Details
Robert Duff, PhD, is manager of Biochemistry, Biopharmaceutical Services at Lancaster Laboratories, Inc. 2425 New Holland Pike, Lancaster, PA 17601; 717-656-2300, rduff@lancasterlabs.com.