On 28 June 2011, the Food and Agriculture Organization of the United Nations declared the Rinderpest cattle plague virus to be the second troublesome virus (after smallpox) that humans have eradicated from the Earth (1). Such achievements herald exciting times both for classical vaccinology and for many new and developing technologies. Here we consider scaling up of vaccines and related hybrid, targeted, and conjugated viral therapeutics that are made through animal cell culture. The vaccine industry is now moving from production in platforms based on whole animals and primary tissues (e.g., embryonated chicken eggs) to cultured-cell–based production (2). This transition began over 50 years ago with polio vaccine development. It is still in progress today, as evidenced by Baxter International’s recent European introduction of influenza vaccine produced by Vero cell culture. And the soon-to-open Novartis plant in Holly Springs, NC, one of the first producing flu vaccine by cell culture in the United States.
Scale-up of cell-culture-based vaccine manufacturing was first accomplished 60 years ago in adherent-cell cultures using large glass flasks. That seminal achievement eventually gave way to arrays of single-use roller bottles, which remain in significant use today. Along with the newer platforms of stacked array flasks and macro- and microcarrier materials, roller bottles complement fed-batch suspension culture as popular platforms for vaccine manufacture. More recent developments in this arena include the introduction of entirely new cultured cell lines and the rapid adoption of large-scale single-use technologies (Figure 1),
Cell Culture Media
Selection and optimization of cell culture media is important to any production-reactor format or cell-line platform for a number of safety, process, economic, and regulatory reasons. The general direction for all platforms is toward use of serum-free media (SFM) and animal-derived-component–free (ADCF) formulations, and the pressure for this transition is somewhat greater for human than for veterinary vaccines.
“Many vaccines are still manufactured using bovine serum or other animal-derived components,” Steve Pettit (director of cell culture development at InVitria) told us. “We have been working with CDC [US Centers for Disease Control and Prevention] and vaccine manufacturers to help them eliminate animal components from vaccine manufacturing.”
Similar pressures exist for chemically defined and low-protein designs, but this is to a lesser degree. Such advanced SFM formulation features have been accomplished for most cell lines used in other areas of bioproduction. But a number of virus and vaccine production formats still require proteins, hydrolysates, animal products, or other media components for optimum performance, which prohibits such designations.
Modern SFM, feed cocktails, and supplements provide for such required functions as cell nutrition, shear-force protection, cell banking, and virus replication in many cell lines and processes. But individual clone-selected or primary cell characteristics require optimization or even redesign of current formulations. For even well-established cell lines, manufacturers’ desire for increased production efficiency often demands customization of feeds and final processes. Growth media for cells used in vaccine and related therapeutic applications begin with basal formulations often developed for each cell line in consideration of other purposes. In many cases, however, special media requirements are imposed by such diverse sources as
- large-scale culture
- the particular production format involved
- the particular virus being cultured
- the degree of product understanding and characterization accomplished to date
- product-specific safety and regulatory constraints
- the relationship between differential cell mass generation and viral product accumulation.
More than a dozen adherent or suspension-adapted cell lines are used by vaccine manufacturers, including both mammalian and insect (e.g., Spodoptera frugiperda Sf9) cells. Mammalian cell lines include Vero (based on African green monkey kidney cells), Madin Darby canine kidney (MDCK) cells, baby hamster kidney 21 (BHK-21) cells, and chick embryo fibroblast (CEF) cells. All such lines were established and can be cultured in a basal medium with an added animal serum. Concerns specific to large-scale and production formats include foaming propensity and remedies; minimum culture seeding density; medium formulation richness and the ability to support concentrated feeds; the molecular weight of active components (e.g., in perfusion applications); and downstream process facility. Virus-specific and product understanding considerations are numerous and include the fact that some virally infected cultures display a heightened or unique metabolite requirement and that some viruses require special culture media activities such as enzyme activation of virion components.
Recent product and process characterization approaches are revealing product heterogeneity in such properties as virus membrane lipid constituents and capsid protein glycomoieties induced by culture procedures and media formulations (3). Ambient culture medium has been demonstrated to influence such production parameters as time of infection, multiplicity of infection and time of harvest. In the vaccine industry, primary drivers in process development and materials optimization include economic considerations such as production efficiency and cost containment, as well as the recent popularity of outsourcing elements of process development to experienced vendors with deep biomanufacturing experience.
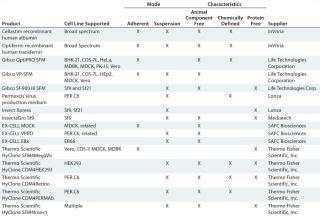
With some notable exceptions (4,5), differential metabolic precursor supply (e.g., amino acids) or special sources of cellular energy (e.g., adenosine triphosphase [ATP]) appear not to be obligate factors for competent virus replication. However, along with such physiological consequences of infection as virus-induced host-cell defense and apoptosis, they have proven to be important events to consider in optimization of most virus yields. So virus and vaccine manufacturers have spent considerable effort in optimization of SFM for production, and a number of commercially available SFM have been developed for many established cell lines as well as for some of the newer and/or proprietary ones such as Vivalis’s EB66 duck embryonic stem cell line and the PER.C6 human cell line from Crucell NV in Leiden, The Netherlands (Table 2).
Reasons for development of such new cell lines are numerous: e.g., safety and regulatory concerns and productivity goals. The ability of a particular virus strain to replicate in certain cell lines does not necessarily directly correlate with its tissue-specific virulence in vivo (6) or the cells suspension or adherent phenotype.
Table 1: Comparing egg-based influenza production with Vero-cell–based production using Hillex IImicrocarriers in Thermo Scientific HyClone SFM4MegaVir media (SoloHill Engineering)
Table 2: Commercially available cell culture media and supplements used in viral vaccine production
Developers should be aware of additional factors specific to cell lines, production formats, and processes when considering SFM for virus-production applications. These include cell attachment requirements and standard raw-material sourcing, shelf life, packaging, and facility/regulatory concerns. In many cases, very similar production media support viral vaccine production from infection-based, stably transfected or transduced cell lines. But some transient-transfection or -transduced platforms require compositional adjustments. Quality by design (QbD) principals apply to culture media as raw materials or components in regulated production (7). Beyond such general guidelines, for example, specific US regulations cover culture media for human ex vivo tissue and cell culture processing (8), and US guidance on biological materials in vaccine production includes such media components as serum and trypsin (9,10).
Production Formats and Equipment
Most cell-culture–based viral vaccine production has historically been performed using anchorage-dependent cultures: The first large-scale virus culture of animal cells was accomplished in the 1950s using an array of 5-L glass (Povitsky) flasks. Roller bottle use was originally developed at Johns Hopkins University for growing large quantities of adherent cells. In addition to increased surface area, the bottles exhibited other advantages over static cultures in preventing gradients from forming in cell culture media and in improving gas exchange with thinner layers of cell culture medium overlay. Disposable plastic roller bottles appeared on the market in the mid-1970s and soon became a mainstay for vaccine production.
Today’s manufacturers can choose from a number of diverse formats supporting either attached or suspension cell cultures. Although many bioprocessors have long-since transitioned to using steam-in-place (SIP), stainless-steel, stirred-tank reactor systems, roller bottles remain in significant use today for legacy processes. They provide robust, well-characterized solutions for some new products as well, for which other platforms have not yet been demonstrated. But the modern bottle shapes, plumbing and closure configurations, culture surface treatments, and housing apparatus would hardly be recognizable to those early culturists (Figure 2).
The next wave of production modes have continued with the disposable theme. These include an engineered extension of the T-flask: stacked-array reactors such as the Thermo Scientific Nunc Cell Factory system from Thermo Fisher Scientific (Figure 2). The scalable system provides a more compact footprint than do traditional roller bottle systems, and it comes in several versions with one to 40 trays and a range of culture media transfer mechanisms, port dimensions and styles, and other equipment to assist in handling.
Microcarriers
Interest in using well-developed and efficient stirred-tank bioreactor systems resulted in further developments for attachment-dependent culture platforms. New low-density matrices that can be easily suspended in bioreactors come as solid or microporous substrates composed of diverse rigid or pliable materials. New manufacturing formats and optimized procedures have emerged to complement them.
Like roller bottles, microcarriers have a long history of use for adherent cell culture. As the move from egg-based to cell-culture–based influenza vaccine production has accelerated, so too has the focus on overcoming some challenges associated with this platform. Mark Szczypka, (president of SoloHill Engineering, Inc.) told us, “Advancements in bioreactor applications — such as new solutions in microcarrier culture — are now providing powerful alternatives to the older methods of vaccine manufacturing.”
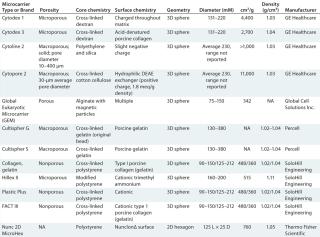
One such accomplishment comes in the ability to culture the large numbers of adherent cells required to seed bioreactors of >1,000-L capacity. Microcarrier-based expansion in bioreactor seed trains has been demonstrated as a viable solution to this challenge. Specialty microcarriers present such characterized and designatable features as cross-linked polystyrene copolymer composition, cationic-modified surfaces, and buoyant densities (Table 3).
Table 3: Microcarriers in vaccine production
For example, SoloHill Engineering addressed recent demands in cell culture processes
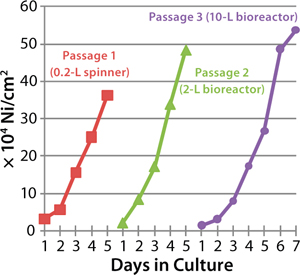
by developing ADCF products such Hillex II microcarriers (11). These specifically charged beads have demonstrated utility for supporting serial passage of many common cell lines as well as success in high-titer vaccine production with ADCF serum-free media (Figures 2 and 3, Table 1).
Other innovations supporting applications in vaccine manufacturing include microcarrier products with ultralow particulate counts, products amenable to gamma irradiation without loss of function, and distribution in convenient and compliant single-use bioprocess containers. Together, these developments support further advancement in vaccine manufacturing, such as the ability to provide single-use bioreactors (SUBs) preloaded with microcarriers for fully integrated systems.
Disposables
Single-use technology has become an accepted component of bioproduction based on animal cells (Figure 4). Both off-the-shelf and custom-designed systems are now in regular use to some extent as part of nearly every production process at contract manufacturing organizations (CMOs) and biopharmaceutical companies, including vaccine manufacturing (12). Drivers for this rapid acceptance and widespread use in such a conservative industry have been well reviewed in recent years. Some of the most important to vaccine production include advantages in facility development and installation time, cost of goods, prevention of lot cross-contamination, rapid turn-around times, production scheduling flexibility, and surge capacity (13).
Disposable components being used in vaccine manufacture include systems for process fluid mixing and storage, bulk material and product storage, filtration, and chromatography, as well as distribution manifolds, sensors, connectors, and production-scale bioreactors (14). Their acceptance by the vaccine industry is evidenced by both the number of companies successfully using them and in published applications (Figure 5).
More than a dozen SUB designs suitable for vaccine production support lot sizes >100 L. Some styles come in the range of 2,000-L capacity. Distinctions among systems offered include agitation and gas-sparging mechanisms, overall dimensions and fluid containment approaches, and applications data and support available. The field remains in a state of rapid development, as evidenced by the recent introduction of an interesting new disposable system involving hollow-fiber bioreactors for virus production in vaccine manufacturing (15). They provide several fundamental advantages not found in other reactor systems (Figure 2). Combining single-use production systems with modular, portable, cleanroom technology promises low-cost, flexible, vaccine-manufacturing facilities that comply with good manufacturing practices (GMPs) to assist developing countries and provide all with swift pandemic response (16).
Driven by technological innovations and such regulatory initiatives as process analytical technology (PAT) and QBD, bioprocess design — including for vaccine manufacturing — is in a period of significant development (17). Increased process understanding, novel analytics, and risk-based methods — and such goals as establishing a life-cycle approach, design space, and critical process parameters — have as much bearing on vaccines as any other products. Just as for other biologics, new guidance and regulations on these topics should help clarify the application of such programs for vaccine manufacturers (18,19).
Virus stock generation techniques such as clone selection and plaque assays used to be performed manually using standard Petri dishes or multiwell plates. Today, a number of specialized procedures using advanced, automated, and even high-content techniques provide product and process developers with increased understanding of virology and bioprocesses. For example, videographic evidence revealed in 2010 that Vaccinia virus infections can demonstrate a “surfing” phenomenon (vigorous repulsion of superinfecting virions) with implications for both in vitro and in vivo infections (20).
Technology Transfer
Initially, virus- or platform-specific culture considerations such as special nutritional or process requirements are addressed at small-scale in process development. Cell culture scale-up in vaccine manufacturing generally follows that of other bioproduction processes, and scale factors for transfer to intermediate levels (from 250 mL to a bench-top reactor) parallel traditional techniques for other applications. For some production formats, however, unique virus-production features need to be considered.
For example, shear forces generated in shake, wave-action, or impeller-driven cultures as well as gas sparging can profoundly affect virally infected cells do to their compromised metabolism and weakened cell membranes. For reasons of convenience — and the tolerance of healthy cells in other bioprocesses to moderate hydrodynamic forces — such forces generated in large-scale agitation and sparging often are not accurately modeled at small scales. For many parameters, however, both micro- and small-scale stirred-tank bioreactor cultures are providing increased control for more accurate scaled-down modeling in process development studies, which allows for more rapid and effective transition into larger production formats (~25 L or greater).
New specialized procedures at such scales include those for such diverse activities as oncolytic virus production for cancer virotherapy, antiviral research, and testing for absorption, distribution, metabolism, excretion, and toxicity (ADMET). For example, three-dimensional hollow-fiber culture is being exploited as an inexpensive and accurate pharmacodynamic model for characterization of viral infection and antiviral approaches (21). With an increasing number of specialized and personalized therapies under development, the concept of “scaling out” by using multiple small reactors — with each reactor designated to provide a patient-specific treatment — instead of “scaling up” to a single large bioreactor system is gaining popularity.
Animal-tissue–based vaccine production is becoming a thing of the past. Entirely new platforms such as plant-based production of virus-like particles (VLPs) present intriguing prospects for the near future. And optimized animal-cell–based methods are currently the gold standard. Many common goals are driving continued development of production-scale, GMP-compliant processes in the most economical and flexible platforms. The number of new therapeutic technologies involving culture of primary or continuous cell lines for gene therapy, vaccine, and new related techniques is large and continues to grow.
Author Details
William G. Whitford is senior manager for the bioprocessing market in Thermo Scientific Cell Culture and BioProcessing at Thermo Fisher Scientific, 925 West 1800 South, Logan, UT 84321; 1-435-792-8277; bill.whitford@thermofisher.com. Alain Fairbank is a biotechnology market consultant, AF Consulting, 158 East 300 North, Logan, UT 84321; 1-435-760-4369; alainfairbank@yahoo.com
REFERENCES