Biopharmaceutical companies generate a vast amount of high-value process data that are typically stored in paper records, data silos, and in the minds of key personnel. This represents valuable corporate knowledge that is often lost or ineffectively used. The manual transcription involved in recording process data necessitates high QA overheads and makes obtaining a holistic view of the processes problematic. It is both time-consuming and costly to support decision-making, root cause analysis, and continuous improvement.
More than 40% improvements in operational efficiency and greater than 70% reductions in QA overheads have been demonstrated using IDBS’ Bioprocess Execution System, which dramatically streamlines process execution, automates analysis tasks, and removes error-prone transcription steps.
An Enterprise Platform for Process Professionals
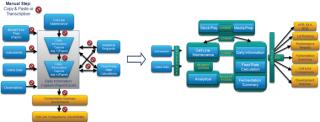
IDBS has optimized and integrated workflows and data at many of the world’s leading biopharmaceutical and contract manufacturing and development organizations. The Bioprocess Execution System is a fully electronic bioprocess execution, data management, and reporting system that supports the entire process lifecycle. It dramatically reduces the time required to develop robust, scalable and transferable bioprocesses; enables more efficient process execution, and reporting; and provides a foundation for operational excellence and quality by design (QbD) initiatives. Interactive reporting tools provide the process insight required to exploit both development and manufacturing data and increase the value of corporate knowledge (Figure 1).
The IDBS Bioprocess Execution System is designed to support the flexibility required by process development, while assuring that the rules and procedures associated with executing validated processes run under good manufacturing practice (GMP) are automatically adhered to throughout. By providing this unified environment for development, scale-up and technology transfer, the whole organization not only benefits from developing and commercializing processes faster and more efficiently than ever before, but it also develops improved process understanding and process quality.
The system also speeds up technology transfer by providing a single source for all product and process lifecycle information, enabling collaboration and communication among internal stakeholders as well as external partners.
Electronic Process Execution, Real-Time Data Integration, In-Line Data Comparisons
Within the Bioprocess Execution System, data capture is driven by a powerful and compliant execution module designed from the ground up for process professionals. Workflow execution templates are intelligently linked to process data and information sources, helping to streamline the execution process and prevent errors. For example, by selecting a media lot number or by scanning the associated barcode, a fermentor setup template will automatically retrieve key information associated with a particular media lot. This ensures that operators have all the information they need in the shortest possible time, while minimizing manual data entry.
Increases of more than 40% in operational efficiency are gained through the automation of analysis tasks, and error-prone transcription steps are engineered out of the process leading to greater than 70% reductions in QA overheads. Key process data are integrated directly into the execution template at the point of use from existing systems such as process data historians, chromatography controllers, LIMS and other laboratory equipment. This means that users are able to keep a near–real-time view of the process without the need to manually collect and analyze all the data from many different systems at the end of a process run. Problems can be identified faster, and root cause analysis can be started immediately.
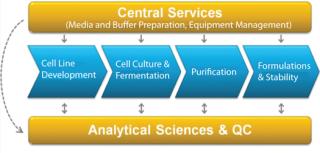
By bringing order to a complex landscape of data and outputs, the Bioprocess Execution System ensures that all data and information are searchable in context, enabling process professionals to more efficiently and effectively look for trends in their data, check process performance or perform root cause analysis (Figure 2).
Reporting and Process Insight
In addition to providing a near–real-time view of key process parameters during the execution phase, the Bioprocess Execution System is able to generate information-rich reports Easy-to-use report templates ensure consistent reporting and comparability between results.
Searching and report generation times are proven to be dramatically reduced when compared with manual processes, enabling rapid delivery of
- Cell line development history reports
- Technology and process transfer documentation
- Campaign summaries
- Annual product reviews
- IND, BLA, NDA
Powerful query tools allow users to quickly retrieve process data and associated context and automatically perform the analysis tasks required to deliver extensive process insight. Examples would be an analysis to compare the performance of a process after the introduction of a new piece of equipment or statistical analysis of cell line performance under changing conditions.
Our Data link functionality allows easy integration with other systems such as LIMS, Process data Historians, ERP, and MES, enabling comparisons to be made with historical manufacturing data, facilitating the integration of online and offline data, and assessment of upstream process changes in relation to downstream effects.
These advanced query and analytics tools provide instant overviews from high-level process data to individual data points. This provides intelligent access to real-time, high-value data across the entire process, helping to support critical process decisions (Figure 3).
#000000;” >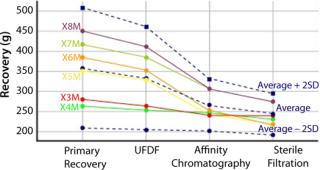
Summary
The Bioprocess Execution System enables organizations to streamline unit operations, reduce QA overheads, and optimize bioprocess workflows, from development to production. Data are stored in context and more effectively shared between groups, significantly speeding up technology transfer, while delivering unparalleled levels of quality and process insight. To find out more about the Bioprocess Execution System visit our website and view our on-demand webinars: www.idbs.com/YearBook.
Author Details
Pietro Forgione is director of biopharmaceutical development at IDBS; +44 208 237 8488; fax +44 1483 595001; pforgione@idbs.com.