Process development for large-scale bioproduction is generally more labor-intensive, time-consuming, and expensive than for comparable nonbiological processes because of the large number of individual processes and potential variables involved. To ensure the future commercial viability of biological manufacturing processes and prevent bottlenecks, it is essential to accelerate development of both upstream and downstream processing, as well as to improve process analytics. This not only reduces time and cost factors involved in design of robust bioprocessing protocols, but also reduces the time to market for new products, offering better returns on research and development investments before patent expiry. The large number of variables and complex processing requirements of biological products are especially challenging for early phase process design, requiring a variety of strategies to achieve rapid bioprocess optimization.
Miniaturization of bioprocess unit operations to the microliter scale offers a cost-effective method of process optimization, reducing material, equipment, and time requirements for development. Adoption of microwell formats also enables ready automation of such processes on a number of commercially available laboratory robotic platforms. Research into miniaturization of bioprocessing has shown that this strategy helps minimize the number of expensive large-scale trials required. The low cost of miniaturized processes also makes conducting those trials far earlier in the product development program economically viable, particularly for biopharmaceuticals.
Parallelizing Upstream Process DesignThe majority of microscale bioprocess studies have focused on microwell systems for investigation of microbial fermentations or enzymatic bioconversions (1), although several recent publications address their use in mammalian cell culture. This format enables both batch and fed-batch studies, providing representative data for predictive scale-up of cell culture processes. The large number of potential experimental variables makes effective bioprocess development for large-scale cultures particularly time-consuming and expensive compared with nonbiological processes. Microwell studies allow parallel investigation of a range of variables for any given cell culture process, dramatically reducing the time required for optimization.
The Department of Biochemical Engineering at University College London (UCL), UK (www.ucl.ac.uk/biochemeng) is at the forefront of this research. It uses the microwell approach in its Bioconversion-Chemistry-Engineering Interface (BiCE) program to speed up development of complex pharmaceuticals and integrate biocatalysis techniques into pharmaceutical syntheses. Led by Professor Gary Lye, the project’s multidisciplinary research team focuses on creation of novel microscale biochemical approaches to accelerate bioprocess design and optimization, including detailed engineering characterization of automated microwell systems.
Characterizing engineering environments within individual wells has been critical to ensuring reproducible, quantitative, and most important, scalable results (2). Well geometry, shaking speed, and liquid fill volume are therefore important parameters for microwell cell culture studies because ineffective gas–liquid mass transfer and poor mixing of liquids within wells are the most common causes of irreproducible or unscalable results. These factors are particularly important for aerobic cultures and fed-batch operations, creating a need for custom microwell systems that effectively mimic large-scale process performance.
Once those issues have been addressed, automated liquid handling platforms (such as Tecan’s flexible Freedom EVO workstations in Photo 1, www.tecan.com) offer reliable and reproducible identification of factors affecting culture performance in microplate fermentations (3). This generic framework acts as a prelude to the predictive and reliable scale-up of optimized culture conditions. Automation offers high-throughput, parallel processing for evaluation of fermentation, bioconversion, and cell culture conditions at an early stage in process development. Compared with traditional methods, this allows investigation of an increased number of variables, at reduced cost, improving overall process understanding.
Photo 1:One particularly useful tool at this stage of bioprocess development is the use of statistical design of experiments (DoE) approaches to further reduce the total number of experiments required. Researchers at UCL have successfully used DoE strategies to optimize a number of biomanufacturing processes, including soluble protein yield. For automated optimization of soluble protein yield from microbial cultures, well geometry, shaking speed, and liquid fill volume were investigated alongside biological parameters using DoE approaches, because these strongly influence oxygen transfer into the wells. Analysis of the model results showed six of the original 10 variables to be important at the screening stage. After optimization, three variables — including microplate shaking speed — were shown to be critical, indicating that oxygen transfer rate is a key consideration for scale-up (4). Compared with standard reference conditions, both the screening and optimization designs gave up to threefold increases in the soluble protein yield, offering a ninefold increase overall. This optimization process predicted a distinct optimum set of conditions for protein expression, which could then be verified experimentally at larger scale (Figure 1).
The high costs of downstream processing in biopharmaceutical manufacture have made development of optimized downstream processes critical for commercial viability. Downstream processing alone can account for >85% of total production costs in many biomanufacturing processes. As such, automated high-throughput techniques are becoming increasingly popular for downstream process development, moving away from traditional experience-based sequential process design. High-throughput screening (HTS) allows a much larger number of variables to be investigated within a commercially relevant time frame, offering far more comprehensive characterization and, therefore, optimization of downstream processes before scale-up.
Miniaturization of individual unit operations has inevitably led to development of complete industrial processes at the microscale. Automated microscale processing on the Freedom EVO platform (Tecan) provides a better understanding of how a fluctuation in a parameter or changing a variable will affect production. Development of pilot-scale processing plants can significantly enhance process performance and reduce production costs. Such scaled-down models enable rapid and comprehensive automated characterization of proposed processes before pilot-scale realization.
The Biomolecular Separation Engineering group at the Karlsruhe Institute of Technology (www.unikarlsruhe.de) has pioneered development of automated pilot-scale bioprocess optimization. The group is closely involved with the biopharmaceutical industry, studying all aspects of downstream processing in biopharmaceutical manufacture, under the leadership of Professor Jürgen Hubbuch. The unrivaled flexibility of the Freedom EVO platform, combined with the high level of space on the deck of the instrument, allowed the group to develop a range of specialist solutions to improve bioprocess characterization, practices that are now being adopted globally by some of the largest biopharmaceutical companies.
To meet rapidly expanding demand for miniaturized HTS processing, several manufacturers are now offering products specifically tailored to these applications. GE Healthcare’s PreDictor plates (www.gelifesciences.com) are one such example (Photo 2), supporting high-throughput process development by allowing parallel screening of chromatography conditions for binding, washing, and elution. These 96-well filter plates are prefilled with BioProcess chromatography media (GE Healthcare) and offer automation of ion-exchange and antibody-affinity chromatography.
Photo 2:PreDictor plates can also be used for determining adsorption isotherms on the Freedom EVO platform. For these experiments, the ratio between protein bound to a chromatographic medium and protein liquid concentration must be varied within a certain interval. Dedicated PreDictor plates with controlled chromatography media volumes of 2 to 50 µL can be used, requiring preparation of only one protein solution. The same volume of protein solution is added to all wells and incubated while mixing for at least three hours. At time zero, all protein will be present in liquid phase (Figure 2), whereas at equilibrium (after three hours or more), the liquid concentration has decreased and protein has been adsorbed on the chromatographic medium, which is given as capacity (Q solid phase). The relationship between protein concentration in liquid at start and at equilibrium equals the phase/volume ratio (Vliquid/Vchrom medium = β), illustrated as dotted lines in Figure 2.
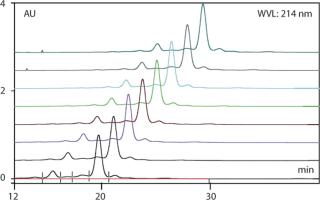
MediaScout RoboColumn arrays from Atoll (www.atoll-bio.com) offer another HTS-specific technology (Photo 3): an automated solution for chromatography media and buffer characterization. These simple, miniature chromatography columns can be prepacked with any commercially available biochromatography resin required, and they are provided in an automation-friendly SBS format of 96 columns. This allows parallel chromatography of up to eight samples in individual columns on the Freedom EVO workstation, with fully automated column preparation, equilibration, sample loading, and regeneration. Column flow controlled by the system’s liquid handling arm through pressure-tight inlets at the top of each column and the Te-Chrom module (Tecan) allows individual fraction collection for subsequent analysis. Elution profiles obtained using RoboColumn arrays can be easily transferred to large scale systems (5) (Figure 3), and automation of the entire chromatography process offers a rapid and reliable mechanism for optimal media or buffer selection, assisting process design.
Photo 3:The high throughput of the automated RoboColumn assay also lends itself to rapid production monitoring applications, such as routine in-process quality assurance of monoclonal antibodies from production-scale fermentation broth. In Figure 4, samples taken directly from a fermentation reactor are loaded onto a row of eight 100 µL CV RoboColumns packed with ProSep-vA high-capacity affinity media. After a short rinse step, bound antibodies can be eluted rapidly using an acidic buffer. Samples are immediately neutralized in the receiver plate by the liquid handling arm of the Freedom EVO workstation before export for high-performance cation-exchange chromatography. Use of automation to accelerate sample preparation for analysis offers significant time savings — reducing preparation time by an order of magnitude compared with manual sample preparation — and offers unrivaled reproducibility (Figure 5).
As well as allowing liquid handling tasks to be performed rapidly, automation can be used to establish rapid process analytics for both process-design and production-monitoring applications. The flexibility of the workstation allows integration of analytical tools onto the platform, offering a much higher throughput capacity for bioprocess optimization. Combining short experimental times with low material requirements enables investigation of a wide variety of variables, reducing costs associated with manual techniques and improving process understanding.
Currently, automated process analytics are limited to basic screening techniques, often using only a fraction of the data generated by HTS platforms. Although they have been used successfully to reduce bioprocess optimization times and improve yields, much of the data are effectively “wasted.” Integration of high-end analytical technologies — such as mass spectrometry (MS), surface plasmon resonance (SPR), and capillary electrophoresis (CE) — onto automated HTS platforms will offer greater insight into bioprocessing reactions. Coupling such techniques with data mining should offer advanced, high-throughput process analytics, improving DoE design without significant additional sample requirements.
Accelerating DevelopmentDetermining critical parameters and optimum conditions for bioprocessing is essential for both process development and production monitoring. A range of innovative methods are now being used to accelerate process development in both academia and industry, with automated approaches playing an increasing role. Automation of process development can offer significant throughput advantages for applications such as sample preparation, separation, and distribution. The platform described here represents a flexible solution for automation of a variety of process development tasks, allowing HTS to be used for design and optimization of bioprocessing protocols before scale-up. Tecan continues to work closely with specialist bioprocessing partners to offer novel solutions for process development in areas such as cell line development, cell culture upscaling, antibody development, downstream processing, and process analytical technology (PAT).
Scientific instrumentation, not for use in human clinical or diagnostic procedures: PreDictor, BioProcess, and ÄKTAdesign are trademarks of GE Healthcare companies; Freedom EVO is a registered trademark of Tecan; Te-Shake and Te-Chrom are trademarks of Tecan; MediaScout is a registered trademark of Atoll; and ProSep is a registered trademark of Millipore. Photo 2 and data in Figure 2 are kindly provided by GE Healthcare, © 2008 (all rights reserved).