Organizations outsource tasks to contract service providers for diverse reasons ranging from internal resource constraints (particularly in virtual or start-up companies) to overflow capacity, or to avoid or delay capital or resource investment. The scope of outsourced work varies from limited tasks such as an outsourced assay to complete process development and GMP manufacturing. In a highly competitive outsourcing market, customers expect successful and timely execution of the outsourced work, but they also seek “added value” to the overall program. Added value can be defined as elements in addition to a contracted service that enhance the economic, scientific, environmental impact, or regulatory status of a project.
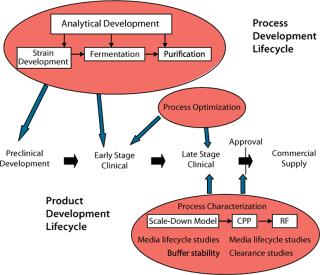
Key ConceptsTwo key concepts in outsourcing biopharmaceutical development and manufacturing projects are project lifecycle and product lifecycle support. The former describes the evolution of a manufacturing process to deliver material for intended preclinical or clinical studies; the latter denotes the ability to support clinical evolution of a manufacturing process and drug substance through all stages of commercialization (Figure 1). This lifecycle approach also could be applied on a smaller scale; e.g. assay development to prequalification status, qualification, and assay validation.

Analyzing the project lifecycle from fundamental development through early clinical stage to commercial manufacturing will reveal many areas where added value can be introduced. First, in silico tools based on bioinformatics and intelligent prediction can aid in designing recombinant proteins to eliminate undesirable characteristics such as aggregation (e.g., in monoclonal antibody production).
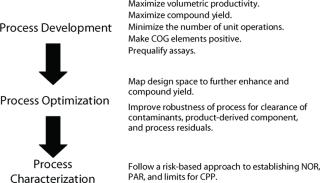
Second is a design for manufacturing approach in which process elements are developed de novo to accommodate the requirements/imitations of GMP manufacturing facilities. Investing in such an approach early will quickly eliminate constraints that might otherwise hinder implementation in a GMP facility, such as inappropriate cell lines or vectors, biomass inoculum train, use of organic solvents, scale, or cycle-time–prohibitive unit operations. Rational process design will place limits on the volume of a process stream and minimize the number of unit operations to maximize throughput and yield. For example, dewatering the process stream early on will facilitate execution, enable use of smaller-scale assets, and minimize raw material and utility consumption.
Cost of GoodsInvestment in strain or cell line development can positively affect cost of goods (COG) by maximizing the production element of the following equations:
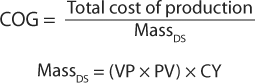
in which MassDS = mass of the drug substance, VP = volumetric productivity, PV = production volume, and CY = compound yield.
This ensures that a recombinant protein is produced in a desired state (soluble or insoluble/inclusions) and cellular localization (cytosolic for bacterial or yeast, periplasmic for bacterial, or secreted for mammalian or yeast). In addition to enhanced production economics, using expression system intellectual property can add value to outsourced process development through familiarity with fermentation process design and performance. This can lead to rapid upstream development timelines and the potential for exploiting defined downstream processing platforms (Figure 2).
Higher volumetric productivities also facilitate efficient use of smaller bioreactor production assets and favor portable manufacturing processes. Designing a manufacturing process using conventional equipment assets and raw materials together with appropriate process scale factors can enable a company to quickly transfer its process in the event of catastrophe or to meet sudden demand (e.g., manufacture of a vaccine or therapeutic to meet a disease outbreak or bioterrorism threat).
Design for manufacturing also incorporates positive cost-of-goods (COG) elements to control or lower batch raw material costs (resulting in a lower total cost of production). Begin by selecting well characterized raw materials of compendia grade from qualified sources to meet process pH, ionic strength, buffering capacity, and polarity requirements. Avoid “exotic” materials to prevent potential supply chain and regulatory problems later in the product lifecycle. Thoroughly define buffer consumption in both development and manufacturing to limit water and solutes (bill of materials) requirements, preparation costs (buffers), and consequently minimize effluent volumes. All of those factors equate to cost savings. Establishing minimum volumes for column sanitization, flush, equilibration, wash, elution, and strip can easily result in >20% savings in materials and preparation logistics, coupled with the added benefit of reduced batch cycle time.
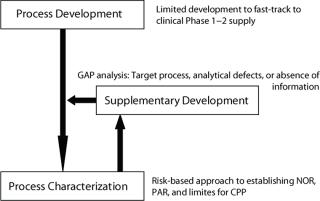
Development HistoryThe product lifecycle is not always a simple progression through clinical evaluation toward commercialization. The high cost of funding clinical supply and trials sometimes necessitates a staged approach, particularly for virtual or start-up companies. Here the priority is fast-tracking the manufacture of Phase 1 or 2 materials and initiating clinical trials as key milestones in support of financing or partnership with medium to large pharmaceutical companies.
Such an approach may result in truncated development programs that use inappropriate technologies for convenience and development speed or insufficient development particularly in the area of process optimization such as mass productivity and yield. The manufacturing process may be established as a working process, but have little manufacturing scale or GMP history.
Demonstrating both clinical efficacy and manufacturing process maturity may be key prerequisites for a drug product partnership. A CMO can bolster manufacturing process robustness with a gap analysis and supplemental development to mitigate risk of failure in mid- to late-stage clinical manufacturing (Figure 3). The CMO also can develop a representative process for scale-down model design and qualification (process characterization).
There are several trends in biopharmaceutical manufacturing that will need to be accommodated within the next five to 10 years. For example, regulatory authorities expect manufacturing processes to be well characterized and have demonstrated robustness. It will become increasingly necessary to map the design space for a given manufacturing process and achieve a greater understanding of the process control boundaries (proven, acceptable, failure) early in the development lifecycle. Achieving these goals will necessitate a greater degree of experimentation, routine use of design of experiments, and automated high-throughput systems.
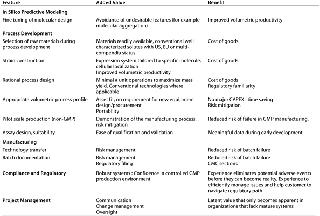
Table 1: Stages in the process development lifecycle and added value
Added value also can be represented in the quality of a process and analytical documentation package. Well written documentation greatly facilitates batch execution thereby lowering risk of error or failure, document review together with ease of abstraction of process data (trending). Although technology transfer procedures focus on successful implementation and execution of the manufacturing process, the value of process flow diagrams, accurate bills of materials, process descriptions, and assay SOPs is significant.
The ability to demonstrate a manufacturing process at pilot scale in a development (non-GMP) environment will also provide some useful information to mitigate risk. In addition to process data, material from such batches could form the basis of an interim reference standard, material for preliminary stability studies, identification of stability-indicating assays and forced stability studies.
Successful technology transfer to manufacturing requires risk management. It also requires a good, predictive understanding of how the process will be implemented in a GMP environment at increased scale. A well-defined technology transfer toolbox and timeline lowers the risk of process or equipment failure and, coupled with mature systems for process education and training, results in fewer operator errors. Well-written and executed batch production documentation has additional benefits such as lessening wasted material, improving observation and data capture, and adding to customer regulatory submissions such as CMC sections and process changes.
Process MaturityOnce a production process is transferred and established in GMP manufacturing, the compliance and regulatory quality systems’ maturity can greatly influence the value of the production batches — over and above the supply of GMP-grade material. For example, initiation and reconciliation of process deviations can either enhance or detract from the documented history of product development. The completeness of a release package to the customer will accurately reflect the capabilities of a CMO. Additionally, a mature CMO will have the depth of experience to support regulatory filings including facility descriptions, writing CMC sections, and meeting with regulators.
Superimposed over the development and manufacturing aspects of project lifecycles is good project management. This benefits the customers through oversight of all project activities, effective communication and budgetary management. Project management can greatly benefit both customer and CMO through responsive actions to changes in scope of work in response to changes in project priorities, timelines, and costs. The value of good quality and project management systems is difficult to measure because the benefits are not always visible or directly measurable. However, under circumstances in which a response to change is required, organizational maturity is critical.
The value of good quality and project management systems is difficult to quantify because the benefits are not always visible or directly measurable. However, under circumstances where a response to change is required, organizational maturity is critical.
Continuity of an outsourced relationship is important to streamline the product evolution lifecycle. However, it is necessary to critically evaluate a CMO’s ability to support the transition from Phase 3 to commercial product. Process characterization and validation require a complement of scientific, technical, validation, and compliance skills with depth and breadth of knowledge and experience. Given the high monetary value of these services, it is crucial that an ongoing, working partnership is established to ensure success.
Outsourced services are based on trust in the technical and organizational capabilities of a CMO and its ability to deliver drug substance product and added value across the customer-service provider relationship.