Biopharmaceutical companies continue to wrestle with how to approach the implementation of process analytical technology (PAT). The accelerated adoption of many new technologies has benefited from the identification of a “killer app,” an early application that enables a readily achieved breakthrough in capability. This can be a totally new capability (e.g., e-mail on the internet) or a major step-change (e.g., 10× or more) in an existing capability. Flashes of inspiration cannot be achieved at will, but we can identify elements of existing processes that are ripe for such breakthroughs. For example, downstream processing capacity increases have fallen far short of the 10× − 1,000×improvements recently achieved in upstream titers due to the inability to increase buffer capacity within existing floor space. The current paradigm for buffer preparation is to premix and store buffers in (relatively large) tanks. A new paradigm is required.
Two candidate solutions are proposed:
-
Reduce demand for buffers by 10× or more, or
-
Change buffer preparation paradigm to the in-line dilution of buffer concentrates consisting of outsourcing the preparation and validation of strongest possible buffer concentrates; storing only maximum possible strengths of buffer concentrates; and using PAT to adaptively and accurately make, validate, and deliver any desired dilution directly to the process.
The first solution implies moving away from the traditional buffer-hungry capture, purification, and viral removal and/or deactivation processes. Such a move is not on the current horizon but should continue to be pursued.
The second solution is eminently possible with existing patented technology but requires an iconoclastic shift in the mind set, a shift that will take place only when the pain of staying with the status quo is greater than that of changing. That point has already passed for most biopharmaceutical companies.
In its “CGMPs for the 21st Century” initiative the FDA wisely recommends that companies adopt a quality assurance system in which a manufacturer takes full responsibility for the performance of its supply chain. Companies should enter into “partnerships” in which the supplier’s workforce effectively becomes an extension of their own. Other highly regulated industries have successfully achieved this, often using audits and “source-inspectors.” This approach frees up floor space and capacity previously dedicated to
-
Raw buffer ingredient procurement, incoming QC/assays, and storage
-
Raw buffer ingredient measuring, 1 × blending, filtering, assaying, and storage in large tanks
-
Cleaning and revalidation of buffer blending and storage tanks
-
The capacity impact of having to remake buffers that fail QC assay.
This floor space is now used to store only buffer concentrates and not the copious amounts of associated water or the multiple different dilutions of the same buffer.
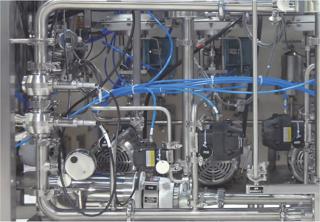
PAT-Based, Just-in-Time Buffer DilutionPortable, PLC-controlled blending and dilution skids of different capacities are available that accurately and repeatedly dilute concentrates, using piped-in WFI, to any desired final blend. This is an excellent application of Pat. Appropriate sensors measure the blend and dilution accuracy in real-time as it is formed. These signals are then used in a two-part control strategy to instantly and adaptively adjust the blend to compensate for any variability in the concentrate feed, and release the exactly correct blend to the process. In this way, continuous validation and real-time release are achieved. The elimination of numerous storage tanks and other equipment enables the 10× or greater increase in capacity from the existing floor space desired for a killer app. As a bonus, this patented approach also delivers buffers to the process to within ± 0.1% accuracy, compared with the ± 5% accuracy of other current buffer preparation approaches. This accuracy permits much tighter control of the processes it feeds, which translates into higher quality, efficiency, throughput, and process understanding.