The demand for biopharmaceutical products is increasing rapidly; however, development timelines of about 12 years leave little time for return on investment before a patent expires. Fast process development is therefore crucial to speed up the market entrance of new biopharmaceuticals.
Until recently, chromatographic process development was restricted to a sequential regime. However, new innovative methods have been developed to perform up to 96 parallel chromatographic experiments on a Tecan Freedom EVO® robotic workstation (pictured, right), allowing for thermodynamic, kinetic, dynamic binding, and elution studies in an automated, parallelized, and miniaturized mode, which saves time, money, and target molecule.
Here we present an overview of the most important chromatographic process development tools on Tecan Freedom EVO® robotic workstations: finite bath thermodynamic studies (1), batch binding in PreDictor™ plates from GE Healthcare, bind/elution studies, and breakthrough/elution experiments with miniaturized chromatographic columns (MediaScout® RoboColums) from Atoll GmbH.
High-Throughput Batch Binding and Elution Experiments with 96-Well PlatesTecan Freedom EVO® workstations are fully compatible with PreDictor plates from GE Healthcare. Fully automated studies focusing on screening chromatographic conditions can be performed using available protocols for both time-dependent and static experiments. The protocols allow investigation into
-
effects of buffer composition, sample concentration, and incubation time on binding capacities
-
effect of buffer composition on efficiency of wash and elution steps on purity
-
resin screening.
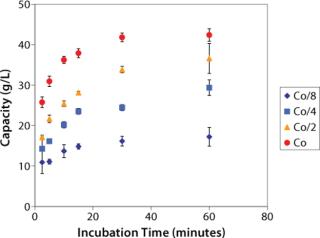
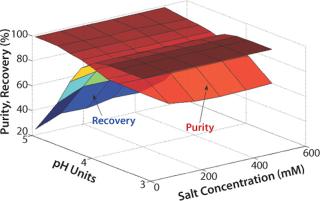
Figure 1 shows an example of a time-dependent study. The results enable estimation of the dynamic binding capacities obtained in a column format under the same binding conditions (2). Figure 2 shows an example of a static study. The results show the effect of a composition of the elution buffer (pH and salt concentration) on purity and overall recovery during elution. Additional information on PreDictor plates can be found in GE Healthcare’s entry in this section of the yearbook: “Screening Loading Conditions on Capto S with a New High-Throughput Format — PreDictor Plates.”
Large-scale chromatographic processes are run in compressed-bed columns, either in bind-and-elute or flow-through mode. Therefore, it is very important to study the dynamic binding and elution characteristics of the target molecule compared with the pollutant molecules for different flow rates, salt types and concentrations, pH values, resins, and so on.
The new opportunity to perform chromatography using any desired resin in correct compression-packed RoboColumns® from Atoll on Tecan Freedom EVO® robotic workstations improves the process information that can be gained at miniature scale, which eases the following scale-up procedure.
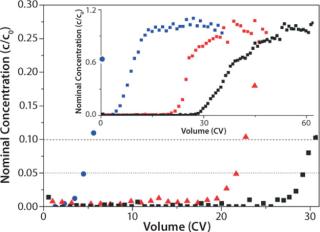
Figure 3 shows a breakthrough experiment, allowing for determination and optimization of the amount of target protein bound under dynamic conditions. The obtained resolution of the breakthrough curves was sufficient to derive binding capacities at 10% as well as at 5% breakthrough for the different salt concentrations.
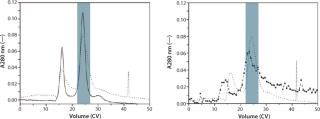
With a restricted parameter space, the elution behavior of a target molecule compared with pollutants was tested. Figure 4 shows the example of an elution experiment with a human growth hormone (hGH) and its precursor (MEAE-hGH).
RoboColumns were packed with Q Ceramic Hyper D using sodium chloride as an elution salt. Separation of the biopharmaceutical compound obtained on Freedom EVO® workstations (Figure 4, RIGHT) is easily transferable to larger scale (Figure 4, LEFT).
SummaryThe combination of Tecan Freedom EVO® workstations and screening procedures evaluating chromatographic process parameters bears the potential to shorten development time of biopharmaceutical purification processes significantly. Optional modules range from simple batch-adsorption techniques to high-throughput techniques (PreDictor plates from GE Healthcare) to miniaturized chromatographic columns (RoboColumns from Atoll). Future uses of such systems to be tackled are in process analytical technologies (PAT) and quality by design (QbD) applications.