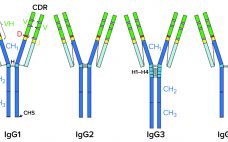
Highâmolecular-weight (HMW) and lowâmolecular-weight (LMW) product variants are critical quality attributes (CQAs) for monoclonal antibodies (MAbs) because they can cause severe immunogenic responses in human recipients. Aggregation is a common problem that can compromise the quality, efficacy, and safety of therapeutic proteins. It can occur at different stages in a biomanufacturing process: during cell-cultureâbased production, downstream process purification, drug-substance formulation, and storage of bulk drug substances or formulated drug products. Hence, the removal and control of MAb aggregates and fragments…
Wednesday, March 16, 2022 Daily Archives
Improving the Performance of Tried-and-True Chromatography Technology
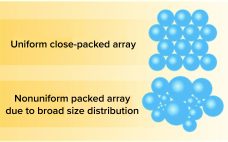
Efficient and effective downstream processing of biopharmaceuticals reduces manufacturing costs and time. Chromatography is the primary purification method for traditional recombinant proteins, monoclonal antibodies (MAbs), plasmid DNA, and viral vectors. Although interest in membrane separation technologies is growing, traditional resin-based solutions continue to be preferred when high-resolution purification is required. Ion-exchange chromatography (IEC)(e.g., cation and anion-exchange chemistries) and hydrophobic-interaction chromatography (HIC) are established bioseparation technologies. Cation-exchange resins are used widely for MAb polishing and aggregate clearance steps. Anion-exchange (AEX) resins…
Mind the Gap: Managing Relationships Between Upstream and Downstream Intensification
Process intensification (PI) describes an integrated framework of strategies to maximize the output of a unit operation, a process, or an entire facility. By implementing PI strategies, biomanufacturers can accomplish their productivity goals by increasing production speeds and titers, reducing facility footprints, and cutting costs. Overall, such changes improve production efficiency and flexibility. Collectively, the biotherapeutic industry has made multiple advancements in intensifying upstream processing. PI strategies include using high-density cell banks, implementing seed-train intensification (n â 1 perfusion), and…
A Missing Link to Achieving Higher Vaccination Rates in Developing Countries
Puerto Rico is leading COVID-19 vaccination efforts in the United States, with 89.7% of adults already fully vaccinated (1). However, many other regions are struggling to gain that level of traction, if any. Less than 1% of people in developing countries are fully vaccinated (2). Because PfizerâBioNTechâs and Modernaâs respective mRNA-based SARS-CoV-2 vaccines still require ultracold temperatures for long-term storage, vaccine distribution in remote locations is arduous without appropriate cold-chain infrastructure. Puerto Ricoâs success in vaccinating its population demonstrates how…
Ligand Binding Assays: Return on Investment Analysis using OctetÂŽ
OctetÂŽ systems enable analytical assessment of biologics in various stages of the development workflow beginning with discovery and early selection to validation, manufacturing and quality control. The instrumentâs configuration and sample plate format coupled with real-time analysis allows for rapid assay method development. In the last couple of years, OctetÂŽ systems have been used by multiple organizations to generate supporting data submitted to various regulatory bodies for the approval of different biologics drug candidates. In addition, Bio-Layer Interferometry (BLI) technology,…
Automation Solutions for the Efficient Integration of Single-Use Equipment
Single-use process technologies have been increasingly incorporated into the manufacturing of biopharmaceutical products over the past two decades. While the reasons for implementing SU systems for biomanufacturing are compelling, there are still some challenges and concerns related to their use. Process automation is often considered one of the weak points of single-use technology. Automation islands are frequent phenomena with SU systems, and integrating the equipment with larger automation systems is challenging. A number of suppliers – like Cytiva, Merck-Millipore, Sartorius,…
Sanofi refills ADC pipeline with Seagen pact
The deal will see Sanofi and Seagen collaborate on antibody-drug conjugate (ADC) candidates targeting up to three cancer targets. Sanofi will leverage its monoclonal antibody (mAb) technology with Seagenâs ADC platform in the partnership. Specific financials are scarce, but Sanofi will make an undisclosed payment to Seagen for each of the three targets as they are selected. The development costs and future profits will be shared between the two companies. âThis collaboration will enable the synergistic combination of molecules and platforms to produce candidate medicines with the potential of bringing renewed hope to cancer patients…
Thermo Fisher shells out $97m to expand clinical research labs
The $97 million investment will expand Thermo Fisherâs bioanalytical laboratory operations into three locations in the Greater Richmond, Virginia region. According to Thermo Fisher Scientific, the decision to expand its clinical research business is in response to increased demand for its services. The investment will see the firm add nearly 150,000 square feet to its Richmond area operations. The laboratories in this location were gained through the firmâs acquisition of contract research organization (CRO) PPD last year. The company is…
J&J taking âdisciplinedâ approach to CAR-T rollout
âCAR-T cell manufacturing is not a finished product,â says J&J EVP Mathai Mammen as he speaks about his firmâs rollout of recently approved myeloma therapy Carvykti. Last month, Johnson & Johnson received the regulatory thumbs up for its chimeric antigen receptor T-cell (CAR-T) therapy Carvykti (Â ciltacabtagene autoleucel; ciltacel), developed with China-focused partner Legend Biotech. The therapy is the second BCMA CAR-T approval â Bristol-Myer Squibbâs Abecma (ide-cel) received FDA approval in March 2021 â and the first cell therapy success…