Scalability remains a critically important topic for biopharmaceutical companies. For conventional protein products, the strategy once was straightforward: Drug makers would scale up, beginning with cultures in flasks and roller bottles to grow enough cells to inoculate laboratory-scale (often glass) bioreactors, then again to pilot- and commercial-scale, stainless-steel, stirred-tank bioreactors. At their highest volumes, such reactors can handle tens of thousands of liters of cells and growth media. If a drug developer did not have the requisite equipment to scale…
Wednesday, March 16, 2022 Daily Archives
Boosting Yield and Preserving Quality: Increasing Production of Induced Pluripotent Stem Cells for Cell Therapy and Regenerative Medicine Applications
Induced pluripotent stem cells (iPSCs) are promising starting materials for several clinical and industrial applications in cell therapy production, drug discovery, and in vitro screening. But as I learned from Maxime Feyeux (cofounder and chief science officer of TreeFrog Therapeutics in Bordeaux, France), such cells are a relatively new option in the toolbox of cell therapy developers. Advanced-therapy products can require controlled proliferation and differentiation of millions or even billions of iPSCs depending on the clinical indication, he pointed out,…
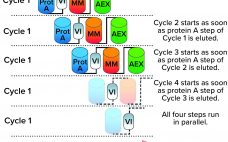
Reducing Downstream Scale-Up Needs: Advances Toward Continuous Downstream Processing
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated…
Scaling AAV Production: Easing the Transition from Laboratory Scales to Commercial Manufacturing
Adenoassociated virus (AAV) has emerged as the leading vector for gene therapy delivery. Compared with options such as lentivirus and adenovirus, AAV exhibits a strong safety profile because it has low pathogenicity and requires a helper virus to replicate. AAV is also capable of long-term gene expression, and it can infect both dividing and nondividing cells (1–5). Developers of advanced therapies have found such advantages to be quite attractive. As of January 2021, two gene therapy products have gained US…
March 2022: From the Editor
As you receive this issue, Informa’s BPI West conference is taking place at the San Diego Convention Center on 14–17 March 2022, with live digital panel discussions the following week. People appear to be cautious but eager to get back to live-conference attendance. BPI’s senior technical editor, Cheryl Scott, will make the journey down there to represent us this year, so if you plan to be there, please take a moment to visit with her. BPI West was the first…
Orphan Drug Designation: Securing the Significant Benefits
When preparing an application for orphan drug designation (ODD) in the European Union (EU), you need to consider key eligibility criteria that are different from those required by the US Food and Drug Administration (FDA). One factor is justifying the “significant benefit” (defined below) of your drug product over existing therapies for an orphan condition. Significant benefit contributes considerably in securing a successful ODD application if the correct approach is taken — as described in two European Medicines Agency (EMA)…
Maximizing European Market Access: Guidance for Young Biopharmaceutical Companies
Achieving efficient and profitable market access for next-generation pharmaceutical products is extremely challenging. The number of drug launches is rising every year, taking competition levels higher with them. And because these novel products tend to be more tightly targeted to smaller patient populations than the “blockbuster” drugs of old, their pricing/reimbursement terms need to be tailored to match. This is especially the case with highly complex biologic drugs, which typically are expensive to research and develop. Below I offer a…
Smart, Real-Time Quality Insights Boost Life Sciences Manufacturing
The COVID-19 pandemic has shone a light on restrictive business processes, information silos, and poor supply-chain visibility in many sectors. In biopharmaceutical manufacturing, for example, difficulties associated with product-quality management have been exposed and starkly felt. However, public healthcare measures over the past 18 months have put physical distance between team members, thereby hampering the usual form-filling, manual sign-offs and spreadsheet-based recordkeeping associated with monitoring traditional manufacturing processes. In some cases, a lack of formal face-to-face discussions in the workplace…
Mycotoxin Risk Determination: Measuring the Potential for Patient Exposure with Antithrombin Alfa Sourced from Transgenic Goat Milk
Antithrombin alfa is a recombinant human antithrombin developed as an anticoagulant treatment for people with hereditary antithrombin deficiency who are undergoing surgical or childbirth procedures (1). Marketed under the ATryn brand name by LFB SA (Les Ulis, France), antithrombin alfa was approved for use in adults by the US Food and Drug Administration (FDA) in February 2009 (2). Antithrombin alfa is expressed in the milk of transgenic goats and purified through a multistep downstream process encompassing both filtration and chromatography.…
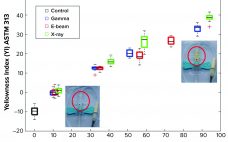
Supplementing Gamma Sterilization with X-Ray and E-Beam Technologies: An International Industry and Academia Collaboration
Ionizing-technology–based industries are growing rapidly around the world. The expansion is driven mostly by the technology’s myriad applications, including polymer crosslinking, medical device sterilization, food pasteurization, and phytosanitary treatment. Ionization also is used in the manufacture of some healthcare products such as medical devices and biopharmaceuticals. Industrial sterilization methods render single-use products and manufacturing components safe and ready for their intended use. ISO11137-1 describes the validation and routine control of a sterilization process for medical devices and mentions the three…