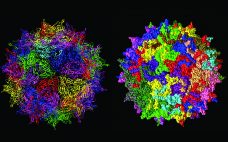
The ability to use adeno-associated viruses (AAVs) for targeted therapeutic payload delivery has dramatically accelerated the development of life-saving gene therapies. Development can further be accelerated by monitoring critical quality attribute parameters like subvisible particles (SVP) concentration early in the development process. Capsid degradation and nucleic acid leakage can both occur under different formulation and storage conditions and contribute to the accumulation of aggregates. Characterizing candidates earlier in the development process is best as it identifies and eliminates inherently unstable…
Monday, February 7, 2022 Daily Archives
Sanofi overcomes CDMO issues with sutimlimab approval
The US FDA approval of Enjaymo (sutimlimab) comes 13 months after Sanofi was hit by a complete response letter citing deficiencies at a third-party manufacturer. The US Food and Drug Administration (FDA) approved Enjaymo Friday, a treatment for patients with rare blood disorder cold agglutinin disease (CAD). The drug, which will be made available in the US at a wholesale acquisition cost of $1,800 per vial, is a humanized monoclonal antibody designed to selectively target and inhibit C1s in the…
Abecma clocks $164m for Bristol-Myers but supply constraints remain
Bristol-Myers Squibb says it expects viral vector supply to ramp up later in the year as third-party and inhouse capacity come online. Bristol-Myers’ Abecma (idecabtagene vicleucel, commonly referred to as ide-cel) was approved in March 2022 as the first cell-based gene therapy for the treatment of multiple myeloma. “Abecma generated revenues of $164 million since its launch in May of last year,” David Elkins, Bristol-Myers’ CFO said during the firm’s fourth quarter call. “Revenues reflect very strong demand for the…
Intellia expands gene editing toolkit with $45m Rewrite buy
The deal will see Intellia Therapeutics add Rewrite Therapeutics’ DNA writing platform and expand its CRISPR functionality. Under the terms of the deal, Intellia, a genome editing firm which uses CRISPR-based technologies will pay Rewrite Therapeutics’ shareholders $45 million in an upfront payment and an additional $155 million in pre-agreed research and regulatory milestones. According to Intellia, the University of California, Berkeley spinout will provide technology that is “highly complementary” to its CRISPR/cas9 and base editing technologies. “Rewrite has developed…
Thermo’s $39bn+ sales driven by 25% y-o-y biopharma growth
Thermo Fisher has increased its guidance for 2022 predicting that COVID-19 related work and the contribution from PPD would drive growth. The technology and services firm announced the revision last week during a full year earnings call when it revealed that full year revenue grew 22% to $39.21 billion. It went on to explain that organic revenue – sales generated by Thermo’s core businesses – increased 17%. It also said the contribution from acquisitions – including contract research organization PPD which…
AES Clean Technology to build $14.2m Pennsylvania plant
AES says it will be able to meet current and future cleanroom demand through the construction of a facility in Lancaster County, Pennsylvania, AES Clean Technology, a provider of cleanroom facilities within the pharmaceutical and biopharmaceutical industry said the facility will expand its production capabilities to match industry growth, especially for clients responsible for the production of COVID-19 treatments and advanced bio-therapeutics. “We see several factors that are driving the decision to add this new capacity. There is a real…