Astrea Bioseparations has a well-established modular program to support customer projects from small to large scales with ligands, adsorbents, and chromatography columns that design purity into each process. Demand for increased productivity in biopharmaceutical manufacturing has placed new pressure on downstream purification operations. For recombinant proteins and monoclonal antibodies (MAbs), such pressure stems from significant gains in upstream productivity, particularly from high titers produced using increasingly efficient cell-culture systems. For viral vectors used in gene and gene-modified cell therapies and…
Tuesday, December 21, 2021 Daily Archives
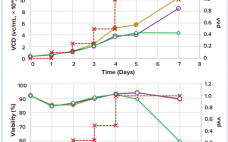
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Tris, a Critical Raw Material: Improving the Quality and Consistency of Supply
ANGUS Life Sciences is the world’s largest supplier of tromethamine buffers and the only manufacturer of the tris molecule based in the Western hemisphere. The company sells directly to biopharmaceutical customers and contract manufacturing organizations as well as to reprocessors who repackage the chemical or process it into different grades and derivatives. After recent expansions in both the United States and Germany, the company now boasts dual-source manufacturing capabilities for its highest-purity tris products and is confident about its ability…
Sanofi to buy Amunix for $1bn
Sanofi has entered into a $1 billion agreement to acquire immuno-oncology firm Amunix Pharmaceuticals. Sanofi said the decision to buy Amunix for an upfront payment of approximately $1 billion and up to $225 million upon achieving specific milestones, supports its plan to increase its contributions to medicines for oncology patients. The French pharma giant will gain Amunix’ propriety technology. This includes its XTEN masking technology platform, Pro-XTEN to identify and develop transformative T-cell engagers (TCE) and cytokine therapies for individuals…
Novavax COVID-19 vaccine wins EMA approval
The European Medicines Agency (EMA) has recommended granting conditional marketing authorization for Novavax’s COVID-19 vaccine, NVX-CoV2373. Novavax’ COVID-19 vaccine, known as Nuvaxovid, is the fifth vaccine recommended in the European Union (EU) for preventing SARS-CoV-2. Additionally, the firm claims it is the first protein-based COVID-19 vaccine approved for use in Europe. Nuvaxovid is made using Novavax’ proprietary nanoparticle technology and Matrix–M adjuvant – to market and the firm received $1.6 billion of US government assistance through the ‘Operation Warp Speed’ program…