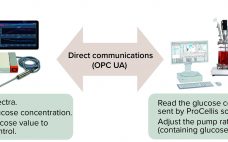
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be ‚Äútested into‚ÄĚ a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
Wednesday, December 8, 2021 Daily Archives
Considerations for Developing Inhaled and Nasally Delivered Biologics
Inhalable drug formulations show much promise for improving biopharmaceutical delivery. But as Ashleigh Wake (director of business development at Intertek) explained to Ask the Expert attendees in September 2021, such formulations necessitate development of complex quality control (QC) analytical methods. Wake highlighted special considerations for characterizing and controlling the stability and potency of inhaled and nasally delivered biologics. Wake‚Äôs Presentation Wake emphasized that nasal and inhaled biological products necessitate QC strategies to control both a protein product and its delivery…
Human Umbilical Cord-derived Mesenchymal Stem Cell Production in Corning¬ģ HYPERStack¬ģ 36-layer Cell Culture Vessels
As more clinical trials are evaluating MSC-based therapies, the demand for more pertinent adherent scale-up tools is likely to increase. Corning¬ģ HYPERStack¬ģ 36-layer cell culture vessels offer a closed system solution for scaling up large quantities of quality, human umbilical cord-derived MSCs. Most importantly, the MSCs expanded in the HYPERStack 36-layer vessel expressed high percentages of CD90, CD105, and CD73, characteristic of MSC quality. The ability to grow large quantities of human umbilical cord derived MSCs with high viability and…
BrainStorm’s cell therapy: Catalent high on NurOwn supply
BrainStorm Cell Therapeutics is eying the potential launch of NurOwn and has selected CDMO Catalent to produce the autologous cell therapy from its site in Texas. Known as NurOwn, MSC-NTF is cell therapy candidate being investigated in Phase III trials for Amyotrophic Lateral Sclerosis (ALS) by BrainStorm. The potential therapy is made from bone marrow-derived mesenchymal stem cells (MSCs) taken from the patient, which are expanded and differentiated ex vivo before being converted into MSC-NTF cells that secrete high levels…
Internal capabilities ‚Äėthe backbone‚Äô of Pfizer‚Äôs gene therapy strategy
With a $500 million facility in North Carolina set to come online this month, Pfizer has lauded its approach to gene therapy development and manufacture. Big Pharma peers Roche and Novartis (via the acquisitions of AveXis and Spark, respectively), have achieved regulatory success in the gene therapy field, and Pfizer hopes to replicate similar achievements in the coming months and years. To get there, the firm is following a ‚Äúthree-pronged strategy,‚ÄĚ according to Robert Smith ‚Äď SVP of Global Gene…