Current demands placed on the biopharmaceutical industry are pushing manufacturers toward process intensification, an approach that modifies unit operations or an entire manufacturing process to optimize efficiency. Three common intensification scenarios in upstream processing are seed-train intensification (usually at the n – 1 stage), concentrated fed-batch production, and dynamic perfusion (at the production bioreactor stage). In downstream processes, intensification strategies typically involve moving from single- to multicolumn chromatography. Biomanufacturers can realize several kinds of improvements from intensified processing, including reductions…
Thursday, October 28, 2021 Daily Archives
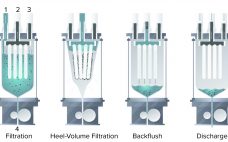
Comparing Single-Use Multicycle Cake Filtration with Depth Filtration: Eliminating the Downstream Bottleneck
Over the past few decades, single-use (SU) technology has increased bioproduction efficiency significantly, especially with the introduction of disposable bioreactors in upstream processing. To keep pace with major developments and increases in upstream capacity, downstream processes also must increase capacity and efficiency. However, cell harvesting and downstream processing continue to present bottlenecks in manufacturing (1). Typical clarification processes are composed of primary and secondary clarification steps, such as centrifugation followed by depth filtration, respectively (1). Two sets of SU depth…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…
Focusing on the Patient Journey Can Increase Access to Lifesaving Therapies
Cell and gene therapies (CGTs) are positioned currently as last-chance, “miracle” cures for patients who have severe illnesses. Such promises require innovation. Despite the cutting-edge science and significant investment that goes into CGT development, fundamental challenges remain, including patient access. The highly personalized nature of autologous-therapy development presents myriad logistical, financial, and manufacturing challenges to ensuring global access to treatment. Understanding a patient’s journey to treatment is vitally important to achieving that goal. Barriers to Cell and Gene Therapy Access…
Regeneron says tech was key to rapid COVID-19 mAb cocktail dev
Efforts to combat COVID-19 were aided by a platform-based approach says Regeneron, which cites its mAb and cell line technologies as key to the process. REGEN-COV, Regeneron’s antibody cocktail reduces the risk of COVID-19-related hospitalization and death by 71 percent and lowers viral loads faster than a placebo according to data recently published in the NEJM. REGEN-COV is a combination of casirivimab and imdevimab. It was developed in less than a year, which is much faster than comparable therapies. Typically,…
Autolomous and Vineti partner to deliver end-to-end solutions in CGT space
Autolomous will combine its manufacturing management systems with Vineti’s Personalized Therapy Management (PTM) platform to form an end-to-end cell and gene therapy pathway. The partnership between Autolomous and Vineti, two technology solution providers for cell and gene therapy (CGT) supply chains, aims to drive the visibility and exchange of information between stakeholders across the CGT value chain to ensure the companies can respond to unpredictable variables that occur with biological processes. According to Autolomous, the integration of the technologies will…
Catalent shells out $230m to expand US gene therapy plant
Catalent will add three additional commercial-scale viral vector manufacturing suites at its gene therapy campus in Harmans, Maryland. The latest investment at contract development manufacturing organization (CDMO) Catalent’s Harmans campus includes the construction of three additional multi-room commercial suites, as well as expanding storage capabilities for ultra-low temperature freezers, just-in-time inventory space,  and its water-for-injection infrastructure. “This latest expansion is to meet growing customer demand and is in anticipation of continued interest and growth in AAV and other gene therapies,”…