Biological drug substances are constituent in a wide range of medicinal products with an even broader spectrum of applications. Those include autoimmune-disease treatments (e.g., for arthritis), vaccines, and recombinant therapeutic proteins (e.g., for cancer treatment). What such products all have in common is that they are manufactured using biotechnology and other cutting-edge technologies. Biologics are not as physically robust as their small-molecule counterparts. Hence, during biomanufacturing processes, these complex molecules present a number of challenges. Some of the typical shared…
Thursday, September 30, 2021 Daily Archives
Creative Formulation: A Useful Approach to Patient-Centered Drug Development
The development of new medicines is a highly regulated process focused on demonstrating efficacy and safety of new products. Although such qualities always will remain the primary focus of drug development, the biopharmaceutical industry gradually has adopted additional design aspects. New approaches can help meet patients’ divergent needs to improve their lives in meaningful ways. Often referred to as “patient-centered” or “patient-focused” drug-product design, such considerations are expected by many experts to become an increasingly dominant part of future drug…
Analysis of Trace-Level, High-Risk HCPs: Proteomics Advances for Preventing Degradation of Polysorbates in Biotherapeutic Formulations
Polysorbate-80 (PS-80) and polysorbate-20 (PS-20) are used widely in formulation of biotherapeutic products for preventing surface adsorption and as stabilizers against protein aggregation (1). Degradation of polysorbates can cause turbidity and potential formation of subvisible particles mainly consisting of poorly soluble hydrophobic free fatty acids (1). Polysorbate degradation is an industry-wide challenge both in biotherapeutics processing and formulation development. The risk of such degradation increases with higher cell densities and greater expression titers in bioprocessing, as well as with higher…
A Holistic Approach: Bridging the Gap Between Suppliers and Biomanufacturers
How can biopharmaceutical manufacturers expect their suppliers to deliver what the industry wants and needs if it doesn’t communicate those desires? Without industry input, biotechnology suppliers are developing technologies “blindly.” Despite their having delivered great value over the years, together the greater community can do better. Aligned industry input is a vital element in the development process. Here we describe how two BioPhorum workstreams focused on drug-product development have worked to facilitate these communications. Last summer, the container–closure integrity (CCI)…
Closed-System Transfer Devices: Collaboration Provides Tools to Guide Compatibility and Stability Testing Strategy
Ever since the first biopharmaceutical product (biologic) was approved in the 1980s, companies have developed protocols and tests to ensure that such products are safe and effective. Biologics are very different from traditional small-molecule drugs, with unique risks inherent to their manufacturing processes. Biopharmaceutical formulations often present as complex mixtures that can be sensitive to heat, light, and many other factors, all of which must be monitored and assessed. However, until recently, developers worked mostly independently, with only their own…
September 2021: From the Editor
Amid the continuing frustrations of this time, we are beginning to see what conducting business in a post-COVID-crisis world will be like. A new survey conducted by our Informa Connect Life Sciences group compares responses with those from similar questions in April 2020 — knowing more than we did before about the impact of COVID-19 around the world. The data reveal insights into how the global life-science community has responded to the crisis, the impact the pandemic has had on…
Preparing for a Virtual Audit
Audits are a vital quality-management tool of the biopharmaceutical industry. Whether the objective is to verify supplier or partner qualifications, contribute to corrective and preventative actions (CAPA), or fulfill regulatory compliance requirements, conducting proactive auditing is key to successful operations. Over the past year, virtual audits —also known as remote or distance audits — have enabled biopharmaceutical companies to meet compliance and quality assurance demands despite COVID-19–related travel restrictions and social-distancing protocols. Notwithstanding the challenges of virtual audits, the benefits…
No More Sleepwalking: New Mindsets for Manufacturing Cell and Gene Therapies at Commercial Scale
Cell and gene therapies (CGTs) offer potential cures to some of the most challenging illnesses of our time. The number of such therapies approved for market is set to surge in the next 10 years (1). Yet current manufacturing approaches are not fit for purpose. Biomanufacturing must adapt to prevent the industry from unintentionally sleepwalking into causing harm to patients. Some urgently needed changes could come with learning about the mindset of the medical-devices industry. Background and Current State After…
Microbiome-Based Therapeutics: Negotiating Key Development Challenges
Microbiome-based therapeutics have evolved significantly in recent years, with several promising candidates advancing through the clinical pipeline. That progress is the result of growing evidence showing that targeting and manipulating the microbiome could improve human health and treat more than 25 conditions by restoring healthy bacterial populations (1). The human microbiome comprises a diverse community of microorganisms, helpful and harmful. It differs by person based on factors such as genetics, environmental influences, diet, and immune function. Microorganisms play a role…
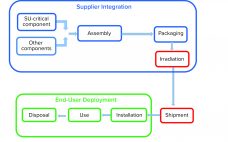
Integrity of Single-Use Systems: Practical Applications and Deployment
Single-use (SU) technology plays an important role in modern vaccine and biologics manufacturing. System integrity, managed by critical process controls, ensures sterility and is a prerequisite for successful leak-free processing. Nonintegral systems cause loss of product, quality, and time; increase costs through investigations; and lead to potential safety problems. The BioProcess Systems Alliance (BPSA) issued a white paper in 2017, Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (1), that describes in detail the strategies for design and…