Frozen storage of bulk drug substances (BDS), including bioprocess solutions, vaccines, blood components, and other delicate process fluids is common in the bioprocess industry. Vessels used to store those fluids must be capable of withstanding long-term storage in extremely cold temperatures (e.g., –85 °C or –196 °C), sometimes for very long time periods. They also must also maintain integrity after repeated thawing and subsequent refreeze. The study below was performed to characterize performance of fluoropolymer bottles with standard and two-piece…
Friday, August 27, 2021 Daily Archives
Freeze Cycle Testing of
Autologous CAR T Cell Manufacturing Using a Semiautomatic, Closed, Modular Workflow: Seamless Transition from Discovery to Clinical Manufacturing
Chimeric antigen receptor (CAR) T cell therapies have advanced rapidly in recent years, with a number of targets in clinical research and several US Food and Drug Administration (FDA)-approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells. Cell isolation, gene editing, expansion,…
Prepared for the Future?
Smart Maintenance Is Key to Industry 4.0
With the rapid advancement of digitalization, new technologies and flexible production concepts have become essential parts of the biopharmaceutical industry, especially for implementing Industry 4.0, the Internet of things (IoT), and predictive maintenance. As an expert partner for digitalization, Zeta supports its customers with new tools for smart maintenance. Swift commissioning helps biomanufacturers complete their projects successfully. That requires trained personnel and maintenance support. Intelligent tools based on digital plant data ensure smooth operation. Smart Maintenance Navigator (SMN) technology is…
Biopharma playing second fiddle to chips and electronics in Samsung’s latest spending spree
Samsung has pledged a staggering $205 billion across its businesses through 2023, but how much of this will actually go to its biopharma businesses? This week, Samsung announced plans to invest 240 trillion won ($205 billion) across its businesses over the next three years with approximately $154 billion of this being injected in its home country of South Korea. The plans drawn up include an astounding 40,000 new hires. But while Samsung’s many business interests include life sciences and healthcare…
Terumo and PhotonPharma partner to develop cancer vaccine
Terumo and PhotonPharma have teamed up to develop Innocell, a therapeutic vaccine for solid tumors. Apheresis services firm Terumo Blood and Cell Technologies and cancer immunotherapy developer PhotonPharma have signed a memorandum of understanding (MOU) to develop Innocell. Innocell is a tumor specific immunotherapy aiming to treat solid tumors. “The therapeutic vaccine is made using autologous tumor tissue taken from the patient as either a biopsy or a surgical resection of the tumor,” Ray Goodrich Co-Founder of PhotonPharma told BioProcess…
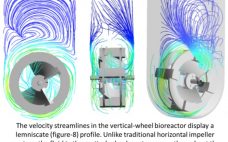
PBS to expand single-use bioreactor tech to feed cell therapy demand
Single-use bioreactor maker PBS Biotech has closed a private $10 million funding round with BroadOak Capital Partners to advance its Vertical-Wheel technology. The funding will be used to expand PBS’ internal operations and capacity, the firm told Bioprocess Insider, to meet the demand and need for its biomanufacturing customers, specifically for cell therapies. Such “investment in manufacturing, engineering and contract services capabilities is required to maintain our rapid growth trajectory in the cell therapy market, we were told. The California-headquartered…