Cold chain distribution is complicated and critical for formulations that must be kept in very cold temperatures in the pharmaceutical industry, since their stability decreases quickly at room temperatures. The World Health Organization (WHO) has reported over 50% of vaccines are wasted and must be disposed of globally every year due in part to disruption of the cold chain distribution and lack the resources to support the ultracold temperature requirements. A possible solution to the existing problem is Hyalo Technologiesâ…
Friday, June 11, 2021 Daily Archives
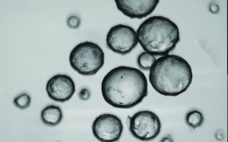
The Five Heresies of Cell Culture: Debunking Conventional Wisdom
Cell culture and bioprocessing conventional wisdom remains a hurdle for the wider adoption of more precise tools. It has been more than 60 years since any real progress has been made towards creating a more accurate and reliable way of performing cell culture monitoring to better understand the effects of things like pH and oxygen at the pericellular level. At SBI, weâre developing optical sensing technologies that unlock the “black box” of cell culture to bring actionable insights to scientists…
Cell-Line Development: Accelerating Antibody Discovery By Monitoring Titer and Glycosylation with the Octet Platform
Cell line development involves the screening of thousands of clones in an effort to find the few optimal clones that are stable, grow as expected, and produce high yields of the bioproduct. The time it takes from engineering an optimal cell line to the production of the target biologic can be prohibitive and may differ from molecule to molecule. While expression-level analysis like titer screening is carried out early, other critical quality attributes (CQAs) such as glycan characterization are often…
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Single-Use Sensors
Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aberâs experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
Simplifying AAV Downstream Process and Product Characterization: A Look at Purification and Analytical Tools
This webcast features: Dr. Julia Baek, PhD, Staff Specialist, Ilaria Scarfone, PhD, MBA, Field Applications Specialist, and Chantelle Gaskin, Field Applications Specialist, Thermo Fisher The optimization of the downstream process for adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity, and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient analytical tools has become evident. In…
Big Pharma collaborative trend to continue beyond COVID
Manufacturing collaboration between traditional rivals will be normal post-pandemic say GSK and Merck & Co., both of which are using their capacity to support fellow Big Pharma COVID-19 efforts. The biopharma space has traditionally been a highly competitive arena. Retaining intellectual property through secrecy, litigation, and other means is crucial to ensure maximum revenues from products developed at high-cost and high-risk. Just look at how the entrance of biosimilars have decimated certain companyâs top line results. But a new era…
Vibalogics plans $50m expansion as viral CDMO demand sits at âall-time highâ
Investment in oncolytic viruses, viral vaccines, and viral vector gene therapies have driven CDMO Vibalogics to expand its capabilities in Germany. Contract development and manufacturing organization (CDMO) Vibalogics will pump $50 million into its Cuxhaven, Germany facility to expand early-to-late phase clinical virotherapy manufacturing capacity. According to the firm, it has seen a growing customer base comprised of Big Pharma, start-ups, and mid-sized biotechs requiring viral vectors for a range of pipeline modalities. âThe work we are doing in support…
Selkirk plans $90m plant to boost US fill/finish capacity
The US needs more pharmaceutical manufacturing capacity on its own soil according to Selkirk Pharma, which has begun constructing a facility in Spokane, Washington.  The planned 145,000 square-foot Washington-based manufacturing plant has been designed for contract manufacturing of injectables drugs, therapeutics, and vaccines. According to Selkirk CEO Patrick Haffey, the intent is âto build much-needed pharmaceutical manufacturing capacity on US soil.â While the firm was founded prior to the pandemic, the realization of the fragility of supply chains in light of the coronavirus pandemic has brought in-country production to the forefront with firms like Avid Bioscience claiming it has made localized…
Novartis to close Swiss stability testing site by 2023
A move to specialized and personalized medicines has led Novartis to plan closure of a Swiss stability testing facility by 2023. Big Pharma has shifted its pipelines away from traditional small molecule drugs to biologics and regenerative medicine over the past 20 years. As such changing needs at Novartis has led the firm to announce it is closing its stability testing facility in Locarno, Switzerland by 2023. âNovartis plans to adapt its product testing capacity in Locarno, Switzerland, to the…