As editors, we are fortunate to have the opportunity to listen to biopharmaceutical developers and innovators discuss the intricacies of their work. Typically, we find that the best discussions come from asking two fundamental questions: What need did you observe in the industry that drove your work, and what technologies would help you do your job better? Over the past five years or so, the answers have shifted. Cell and gene therapy (CGT) innovators are focusing on increasingly complex diseases…
Monday, April 26, 2021 Daily Archives
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
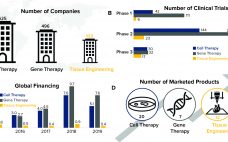
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
The Difficulties of Manufacturing Cell and Gene Therapies At Scale
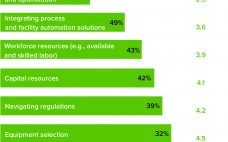
From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…
Chinese CDMO sector growing but unlikely to make global impact
China’s contract manufacturing space is growing at over 15% year-on-year but only WuXi Biologics has seen success in attracting global clients, says BioPlan Associates ahead of CPhI Discover. Third-party drug manufacturing is relatively new in China. Only since 2015 has a market authorization holder (MAH) system been in place, opening the doors through further reforms for commercial contract development and manufacturing organizations (CDMOs) to operate in the country. In 2019, China approved Hodgkin’s lymphoma drug tislelizumab – the first CDMO-made…
BMS to build fifth cell therapy plant in response to CAR-T success
After recent CAR-T cell therapy success, Bristol-Myers Squibb will build its fifth advanced therapy manufacturing plant in Leiden, The Netherlands. The biopharma giant has invested in its fifth cell therapy manufacturing plant in Leiden, Netherlands. The plant will be its first in Europe alongside global manufacturing partnerships. The investment is part of its continued commitment to growing its cell therapy franchise and patients with haematological cancers. “At the heart of Bristol Myers Squibb’s commitment to cell therapy is our continuous…
Bayer confirms $200m Berkeley cell therapy facility
The Cell Therapy Launch Facility will support Bayer’s growing advanced therapy ambitions and comes in addition to a $1.2 billion investment in the Californian campus. Bayer has confirmed it is expanding its cell and gene capabilities through the addition of a Cell Therapy Launch Facility, which is set to join a Cell Culture Technology Center (CCTC) and Cell & Gene Therapy labs coming on-line later this year at its 46-acre campus in Berkeley, California. “The Cell Therapy Launch Facility is…