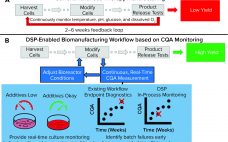
Cell-based advanced therapies are changing modern medicine dramatically. Immunotherapies such as chimeric antigen receptor (CAR) T-cell therapies are treating different forms of cancer. Gene therapies are reversing the course of inherited diseases, and tissue-engineered medical products are restoring, maintaining, and replacing damaged organs (1–4). The development of new advanced therapies is booming. As of January 2020, the US Food and Drug Administration (FDA) has reported more than 900 investigational new drug (IND) applications for cell and gene therapy products. However,…
Tuesday, March 9, 2021 Daily Archives
Using Prior Knowledge to Estimate Long-Term Variation
A reasonable estimate of long-term variation for a biopharmaceutical product critical quality attribute (CQA) can be challenging to justify, especially in the early stages of a product’s lifecycle when only limited data are available. However, if the combination of product and analytical method reasonably can be matched with historical data, prior knowledge can provide an estimate of a value. This variation estimate could be used to assist in risk assessments related to continued process verification (CPV) activities, including control charting…
Ask the Expert: FPLC Column Selection Considerations
On 10 November 2020, BPI presented an “Ask the Expert” webinar with Dan Yukon (head of North American and global SNAP product sales at Astrea Bioseparations) on considerations for selecting analytical fast-protein liquid chromatography (FPLC) columns. With many options on the market, deciding which type and brand to use can be difficult. To help take out the guesswork, Yukon addressed a number of topics, including pressure and volume considerations; column configuration; materials of construction; frit type, design porosity, and mounting;…
Ask the Expert: Centrifugation Guided By Optical Sensors Enables Efficient, Reagent-Free Cell Separation
Ben Josey, PhD (field application scientist at Corning Life Sciences), joined BPI on 3 December 2020 to deliver an “Ask the Expert” presentation about using optical-sensor–guided centrifugation for cell therapy development. Cell-separation techniques fall into four basic categories: filtration, centrifugation, affinity purification, and emerging methods such as microfluidics and acoustofluidics. Selecting the technology best suited to an application requires careful balancing of method precision and process efficiency, the latter of which includes factors such as batch size, time, labor, and…
Ask the Expert: New and Improved Analytical Methods for Traditional and Unique Modalities
On 10 December 2020, BPI presented an “Ask the Expert” webinar with Jason Sterling, PhD (principal scientist and project director in analytical and formulation resources), and John Rockwell (group leader) of Catalent Pharma Solutions. Biophysical characterization is critical to understanding the makeup and behaviors of biologic therapies and vaccines both early in development and throughout scale-up for manufacturing. As biologics become more complex in structure and as scientists improve their understanding of the effects of structure on stability, efficacy, and…
Ask the Expert: Developing Strategic Analytical Programs for Therapeutic Peptides
Ashleigh Wake began her 15 October 2020 “Ask the Expert” presentation by pointing out that peptide products are manufactured in a “regulatory vacuum.” Peptide-product developers must be strategic in designing characterization and quality control (QC) programs. Wake reviewed available methods and explored key considerations for developing phase-appropriate analytical controls. Wake’s Presentation Because peptides overlap small- and large-molecule drugs in size, regulatory expectations differ by product size and clinical indication. Thus, analytical programs should be designed around critical quality attributes (CQAs)…
COVID-19 As a Catalyst for Changing Orphan-Drug Regulations
A long-awaited (and for months withheld) evaluation of the European Union’s Orphan Drug Regulation (ODR) finally reached the interested public in August 2020 (1). Its publication during the public consultation of the European Commission’s “Pharmaceutical Strategy” was well planned because the latter discusses policies on access, availability, and affordability of new medicines. However, the ODR evaluation shows not only that the intentions behind the legislation have not been fulfilled, but also that its generous incentives (extension of market exclusivity and…
Going Dutch: Intravacc paves way for in-country vaccine production
CDMO Intravacc has started a concept design for a so-called ‘Multi-Purpose Vaccine Production Plant’ in response to COVID-19. Dutch contract development manufacturing organization (CDMO) Intravacc has signed a letter of intent alongside Bilthoven Biologicals, Poonawalla Science Park and Alt Foundation to begin developing a Multi-Purpose Vaccine Production Plant (MPVPP) at Utrecht Science Park, Bilthoven. According to Intravacc, the coronavirus pandemic has exposed the vulnerability of the Netherlands regarding the development and manufacturing of vaccines for its own population. The firm…