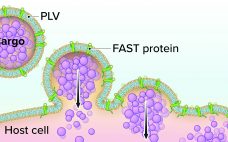
Although vaccine platforms based on messenger RNA (mRNA) are enjoying the limelight in the wake of emergency authorizations of products from Pfizer–BioNTech and Moderna, DNA vaccines are poised to make their own commercial debuts soon. The World Health Organization (WHO) reports that six of the 48 candidate vaccines against SARS-CoV-2 that remain in clinical trials are DNA-based products, as are 14 others in preclinical study (1). I spoke with Hong Jiang (cofounder and chief operating officer of Aegis Life, Inc.)…
Friday, February 5, 2021 Daily Archives
Cold-Chain Validation: Emerging Vaccines for COVID-19 and Beyond Require More Extensive Evaluation
In the wake of fast-track approvals for Pfizer’s and Moderna’s respective SARS-CoV-2 vaccines, now begins the largest immunization campaign in world history. Its success will depend not only on the products’ safety and efficacy, but also on several mass-distribution programs requiring significant cold-chain infrastructure. The public has become acutely aware of the Pfizer vaccine’s demanding cryostorage specifications, generating considerable anxiety about how mass distribution will happen. Behind the scenes, however, cold-chain engineering companies such as Modality Solutions have worked alongside…
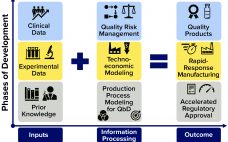
Enhancing Vaccine Platforms: Computational Models Accelerate Development, Manufacturing, and Distribution
Pandemics such as the current COVID-19 outbreak pose tremendous healthcare and economic challenges. Vaccines hold promise for controlling pandemics; however, substantial challenges come with pandemic-response vaccine development, manufacturing, distribution, and administration. To address those now, many companies are using rapid-response vaccine-production platform technologies. Computational modeling tools could help further accelerate development of those technologies, increase production and distribution efficiencies, and reduce costs and risks once vaccine platforms are fully developed and validated. To those ends, a set of modeling methodologies…
Advanced Analytics to Accelerate Development of Genetic Vaccines
Biopharmaceutical companies are racing to develop vaccines that mitigate the COVID-19 pandemic, taking a wide range of vaccine-development approaches that include traditional modalities and cutting-edge technologies based on DNA and RNA. Vaccine developers are leveraging robust manufacturing concepts and integrated processes to shorten timelines. Advanced analytics also are playing a critical role in ensuring the safety and efficacy of those emerging vaccines. A New Wave of Vaccines Vaccines based on attenuated viruses entail development timelines ranging from four years to…
Reacting to a Pandemic: Innovations in Vaccine Development
Traditionally, viruses for vaccines have been grown in embryonated hen eggs. But new challenges introduced by the COVID-19 pandemic have encouraged and catalyzed innovations in the field of vaccine development. The biopharmaceutical industry has recognized an advantage in mammalian cell-culture systems as promising alternatives to egg-based vaccine production. Cell lines can be cultured to large quantities in bioreactors, allowing for much shorter lead times, a more controlled production process, and a higher grade of reproducibility through standardization. In this article,…
Workforce, tax incentives and US access attract more biotechs to Puerto Rico
CytoImmune Therapeutics and Biosimilar Sciences will invest a combined total of $228 million to set up operations in Puerto Rico. The plan – which was announced by Invest Puerto Rico last week – will see the firms perform novel biologics and cell therapy research and development and hire around 400 employees. A CytoImmune spokesman told us “We chose Puerto Rico due to talented workforce, experienced Pharma service providers, tax incentives, excellent access to US and worldwide shipping, US manufacturing. He…