With this issue, BPI celebrates its 19th birthday. I have pretty much run out of words to talk about the year we all just endured and the changes we’ve had to make in our lives to sustain our families, businesses, and overall mental and physical health. We editors want to respond appropriately to the complex issues currently facing the biopharmaceutical industry. But with so many technologies advancing so quickly, it’s difficult for a (mostly) monthly publication such as ours to…
Thursday, February 4, 2021 Daily Archives
Announcing the Winners of the 2020 BPI Readers’ Choice Awards
In the fall of 2020, BPI premiered its Readers’ Choice Awards program. Concentrating on articles published from September 2019 through June 2020, with rankings based on digital engagement, BPI staff identified the most popular articles within each of six categories of bioprocess coverage. You ranked those articles in terms of innovativeness, applicability, and presentation. We’re pleased with the turnout and delighted now to congratulate the winners, who were announced first online. Analytical Award BPI congratulates the winner of our Readers’…
The Power of Industry Collaboration: Driving Harmonization of Regulatory Requirements
The biopharmaceutical industry continues to develop advanced manufacturing processes, systems, technologies, and facilities. Regulations play a significant role in assessing and approving marketing authorizations for drug products that are submitted for approval. The industry and regulators together should form a coordinated, streamlined process that delivers much-needed medicines around the world. Global Complications The complex global nature of biopharmaceuticals sometimes means that progress is not always as smooth as it could be. One main reason is that regulatory agencies often differ…
The Green Imperative: Part Two — Engineering for Sustainability in Single-Use Technologies
In BPI’s June 2020 issue, the first installment of this series introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (1). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the…
Top Trends in Biomanufacturing
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (1). Top trends from this report are highlighted…
Fetal Bovine Serum Source Countries: Comparing Regulatory Animal Health Infrastructures
The motto of the European Serum Products Association (ESPA) — “Serum Saves Lives” — reaffirms the essential role that animal serum plays in cell-culture–based research and applications to protect the health of both human and animal populations. Animal serum, especially fetal bovine serum (FBS), needs to be available in abundant supply and at affordable prices to medical and veterinary facilities all over the world. With one billion cattle globally, the supply of FBS should be plentiful, but not all countries…
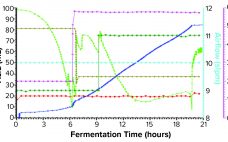
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
Removing Oligomers of a Recombinant Human Therapeutic Hormone:
Evaluation of Chromatographic Options for Effectiveness
Aggregation is a common cause of protein instability, which renders a biologic product unfit for therapeutic use. Sometimes it is difficult to purify monomeric proteins from oligomers because of similarities in their isoelectric points (pIs). Proteins such as hormones have pI ranges similar to their oligomers and thus can be difficult to separate out using a conventional polishing chromatographic step such as ion exchange. With those pI similarities, removal of oligomers to a considerable extent by ion exchangers can compromise…
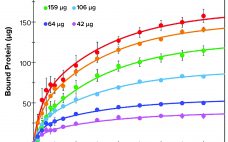
Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (1). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (1–3). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX)…
Wacker acquires plasmid DNA manufacturer Genopis for $39m
The German chemical company Wacker takes over Genopis from Helixmith, adding plasmid DNA to its CDMO offering. In 2018, Helixmith (formerly ViroMed) and private equity firm, Medivate Partners created Genopis. Wacker paid $39 million in cash for Genopis with the potential for further payment depending on performance-based payments under a so-called earn-out model. The deal is expected to close in Q1, 2021. Wacker will continue Genopis’ current customer relationships as a contract development manufacturing organization (CDMO) for plasmid DNA (pDNA).…