For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
Wednesday, September 23, 2020 Daily Archives
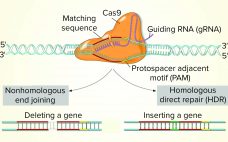
Use of CRISPR and Other Gene-Editing Tools in Cell Line Development and Engineering
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel ‚Äúdesigner‚ÄĚ CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
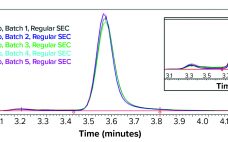
Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
From flu jabs, to mAbs, to COVID collabs: BioNTech nabs Novartis plant and labs
BioNTech is buying Novartis‚Äô facility in Marburg, Germany to support the manufacture of its mRNA vaccine candidate against COVID-19. Financial details of the acquisition have not been divulged, but the GMP facility in Marburg (about 50 miles north of Frankfurt) will be used to increase manufacturing capacity for BNT162b2, a Phase III messenger RNA (mRNA) vaccine in development with Pfizer against COVID-19. The Novartis facility houses equipment to produce recombinant proteins, and cell and gene therapies ‚Äď as well as…
Moderna says ‚Äėsimple‚Äô mRNA process allowed speedy COVID vaccine scale-up
The cell-free and enzymatic nature of its mRNA technology has allowed Moderna to quickly build a relatively small network capable of supporting commercial manufacture of its COVID-19 vaccine. On Monday, President Trump named Pfizer and J&J as leading the way in bringing a coronavirus vaccine to market. But also speaking Monday, Tal Zaks, chief medical officer at Moderna Therapeutics, believes it will be his firm that will be the first to bring both a vaccine to market and ‚Äúto deliver…