By the time you read this, I will have officially called it a career at BioProcess International. Over 18 years — where did the time go? In many ways it feels wrong to be writing this — too soon, with too much still to do. But deep down, I know the timing is right. Professionally, it’s time to turn the reins over to the competent, experienced, and tireless staff you already know — either personally or through the pages of…
Tuesday, September 22, 2020 Daily Archives
September 2020: From the Editor
I’m writing this from the height of our pandemic summer, and you’re reading it in the fall — in this strange year of time flying while seeming to stand still. We editors have worked from home for years, so our lives haven’t changed as much over the past few months as yours might have. Our Informa conference organizers, however, have seen their jobs transform radically. They’ve managed to pivot deftly toward virtual events, and I hope you’ve enjoyed some of…
Unraveling the Complexities of Technology Transfer
In the biopharmaceutical industry, technology transfer refers to transfer of any process, together with its documentation and professional expertise, between development and manufacture or between manufacturing sites (1). This operation is common in the biopharmaceutical industry for a number of structural reasons. They include the dichotomy between small, innovation-based drug companies and large ones able to conduct late-phase clinical development and endowed with manufacturing capacity; the high capital cost of biopharmaceutical plants, which makes contract manufacturing attractive; and the need…
Development of Patient-Focused Commercial Specifications: Understanding Clinical Relevance and Criticality of Quality Attributes
The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing…
Developing Advanced-Therapy Products Through Global CDMOs
Tremendous growth in the cell and gene therapy (CGT) industry is driving unprecedented demand for manufacturing services. To be sure, advanced-therapy developers increasingly are choosing to install in-house capabilities. Doing so can offer companies greater control of their processes, timelines, and budgets than they might have when outsourcing products (1). But industry experts agree that contract development and manufacturing organizations (CDMOs) will remain integral to CGT manufacturing and commercialization (1, 2), especially with veteran contract partners scrambling to acquire CGT…
Toward a Roadmap for Cell-Free Synthesis in Bioprocessing
Cell-free synthesis (CFS), also known as cell-free transcription and translation, supplements cellular components (either a cell lysate or purified recombinant elements) with nucleotides, amino acids, metabolic intermediates, and salts to produce a nucleic acid or protein from a genetic template added to the reaction. This exciting technology has seen a substantial increase in both academic and commercial interest over the past decade (1). Interest stems in large part from the potential to democratize access to the machinery of biology by…
Product Quality Attribute Shifts in Perfusion Systems, Part 1: Identifying Shifts When They Occur
Perfusion cell culture processes are continuous, with fresh media continuously added and spent media (harvest) removed simultaneously through a cell-retention device (Figure 1). To maintain specific bioreactor cell density, cells are removed periodically as cell bleed or discard. Perfusion systems offer a number of advantages over batch and fed-batch culture modes such as lower capital costs and an ability to support higher cell densities with better viability over longer manufacturing campaigns requiring shorter turn-around times. However, perfusion systems require complex…
Microbial Expression and Purification: One Company’s Historical Perspective
Since the dawn of the recombinant DNA era in the 1970s, New England Biolabs (NEB) has been integrally involved in expressing and purifying proteins, both for its own research interests and for biomanufacturing processes. In 1978, the company began screening microorganisms for restriction enzymes. Our scientists remember the challenges met in purifying limited amounts of restriction enzymes and other proteins from native organisms isolated from the environment. The efforts of those scientists to clone, overexpress, and purify restriction enzymes from…
Dissolved Oxygen Control Tuning for Cell Culture Applications
Proper tuning of dissolved oxygen (DO) controller proportional integral (PI) values is essential for optimal cell culture performance in a bioreactor. When DO-PI values are optimized, gas flows are smoothed, and foaming and cell stress are reduced. Traditionally, this tuning has been performed by using nitrogen gas to purge oxygen from a test solution, thus simulating oxygen demand. That method has several drawbacks, however. First, nitrogen gassing cannot simulate the high demands of high-density fermentation. Second, nitrogen competes with other…
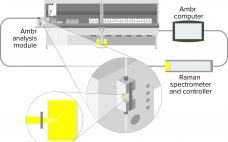
Novel Integrated Raman Spectroscopy Technology for Minibioreactors: Accelerating Raman Model Building for Cell Culture Monitoring and Control
Raman spectroscopy is used widely in biomanufacturing as a process analytical technology (PAT) for monitoring analytes such as glucose and lactate (1). Predictive Raman models also can be used to control glucose concentration in cell cultures (2). The technique is becoming more popular for pilot- and manufacturing-scale bioreactors, but it only recently has been studied with minibioreactors for measuring analytes and producing predictive Raman models for feedback control (3) thanks to advances in integrated technology for automating sampling, analysis, and…