The addition of cryogenic freezer systems firm MVE Biological Solutions will boost Cryoport’s position in the temperature-controlled cell and gene therapy logistics space. Cryoport, a temperature-controlled supply chain services firm for the life sciences, has agree to buy MVE Biological Systems for $320 million with the deal expected to close by the end of the year. MVE is a subsidiary of Chart Industries and will bring Cryoport a greater presence in the cell and gene therapy services space, broadening its…
Thursday, August 27, 2020 Daily Archives
Stainless-Steel and Single-Use Systems: Observations from COVID-19
Justin Carbungco, associate director, small-scale commercial manufacturing, Samsung Biologics Carbungco began with what he described as a lingering question: Which is better, single use or stainless steel? He focused on cost comparisons for stainless-steel and single-use systems, including some factors for companies considering clinical and contract manufacturing. Implementation of single-use systems is estimated to grow to ~US$33 billion by 2027. The year-end growth rate as of 2020 will be about 10.85%. For biopharmaceutical manufacturers, most single-use systems are implemented at…
Get to IND Faster: Accelerated, High-Performance Cell-Line Development
John Gill, director of cell-line development, Samsung Biologics Samsung Biologics has expanded its capabilities with a goal of providing end-to-end biomanufacturing solutions. The company has offered both drug-substance (DS) and drug-product (DP) manufacturing services since its inception. Across three sites in South Korea, the company offers 364,000 L of bioreactor capacity, including units for n – 1 perfusion cell culture. To those capabilities Samsung has added contract research support for biosafety testing, standalone quality control (QC) services, and a novel cell-line development…
An Innovative, Closed, CAR T-Cell Therapy Platform Process to Streamline Manufacturing Approaches with Great Predictability
Tatiana Golovina, senior director, cell therapy process development, WuXi Advanced Therapies Golovina described recent developments at WuXi Advanced Therapies, a unit within WuXi AppTec that specializes in developing and manufacturing cell and gene therapies (CGTs). Until recently, the Advanced Therapies business operated over three sites at the US Navy Yard in Philadelphia, PA. Those facilities focus on CGT clinical manufacturing, commercial manufacturing for autologous and allogeneic cell therapies, and late-stage and commercial production of viral vectors and gene-mediated cell therapies,…
From Target to Hit: The WuXi HitS Platform
Alex Satz, senior director, DEL strategy and operations, WuXi AppTec Satz talked about WuXi AppTec’s launch of the HitS (Hit Success) business unit, designed to serve client needs in early stage drug discovery. WuXi’s goals are to provide more and higher-quality hit molecules against targets of interest and to help set the stage for follow-up medical-chemistry optimization. The unit works with all protein target classes, including solubilized membrane proteins and challenging protein complexes. A core strength of the HitS business…
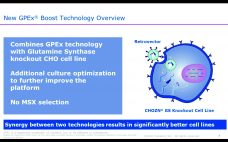
GPEx Boost: A Novel Approach for High-Expressing CHO Cell-Line Engineering
Gregory Bleck, PhD, global head of R&D, biologics, Catalent Catalent’s GPEx Boost technology, based on the company’s GPEx technology, is used for engineering high-expressing Chinese hamster ovary (CHO) cells for pharmaceutical production. Using replication and retrovector technology, genes are inserted into mammalian cell lines. The process requires neither antibiotic selection nor use of toxic compounds for gene amplification to make genetically stable cell lines, so stability testing can be kept off of the critical path for some programs. To date,…
CRISPR Library Screens for Oncology Target Discovery at WuXi AppTec
Yong Cang, professor of cancer cell biology, Shanghai Tech University (China) and scientific advisor, WuXi AppTec Because cancer is a genetic disease, Cang observed, researchers are trying systematically to identify genes that enable cancerous propagation. That often requires studying how familiar genes are expressed in vitro. But such cellular behavior can change under different experimental conditions: e.g., when cells are cultured in two- or three-dimensional bioreactors and when studies involve therapeutic compounds or T cells. Cang suggested that researchers might be…
Solutions for Rapid Development and Manufacturing Responses to COVID-19 and Other Public Health Threats
Richard W. Welch, PhD, vice president of development services, Emergent BioSolutions Welch focused on elements of pandemic preparedness. Emergent has a history of working with the US government on bioterrorism and biodefense preparedness, with a focus in recent years on developing platform-based responses to emerging disease threats. Emergent is focusing on therapeutics and vaccines to respond to COVID-19. The company’s therapeutic business unit uses the company’s internal hyperimmune process to generate therapeutics from the plasma of recovering donors, leveraging an…
A Holistic and Integrated CMC Development Approach: Committing to Quality, Reliability, and Speed
Jerry Yang, senior vice president and general manager, HJB International Yang introduced his company, HJB, as an integrated contract and development manufacturing organization (CDMO) with business centers in Boston, San Francisco, and Shanghai. The manufacturing site in Hangzhou City is about an hour-long drive from Shanghai City. The company’s mission is to maintain a global quality business standard, developing innovative technology to balance low costs with high quality, and to serve as a flexible, efficient, and reliable partner. Integrated platforms…
New Business and Funding Models to Accelerate Development of Life-Preserving Therapies
Jonathan Freeman, PhD, founder and COO, Anthos Therapeutics, and senior advisor, Blackstone Life Sciences; and Abdelaziz Toumi, PhD, head of Ibex design and development, customer solutions, Lonza Pharma & Biotech Freeman spoke about the history and philosophy of Anthos and its core program, focusing on specific needs of virtual biotechnology companies particularly from an investor standpoint. Anthos was created after the formation of Blackstone Life Sciences, in collaboration with Novartis. Blackstone’s goal is to find innovative financing approaches that enable…