The deal expands upon a 2018 collaboration and comes as Translate Bio builds out its mRNA vaccine production facilities in Massachusetts. In 2018, French pharma giant Sanofi teamed up with Translate Bio to develop messenger RNA (mRNA) vaccines for up to five infectious disease pathogens for an initial three-year period. This week, the firms have announced the collaboration will be expanding. mRNA vaccines work by delivering a nucleotide sequence that codes for the proteins that pathogens use to cause disease.…
Wednesday, June 24, 2020 Daily Archives
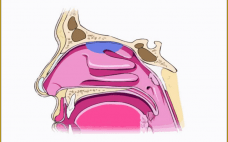
Translating Inhaled and Nasal Technologies for the Delivery of Biologics
This webcast features: Mark Parry, Technical Director, Intertek Inhaled and nasal delivery platforms have specific applications outside of their traditional uses for asthma/chronic obstructive pulmonary disease (COPD) and seasonal rhinitis/sinusitis: They can offer real advantages for the delivery of therapeutic biologics. During this short presentation, Intertek’s Technical Director, Mark Parry, will provide an overview of currently available technologies and successfully marketed products, with a look at the development challenges that might be encountered — and the solutions that are available…
Cytiva bolsters CDMO business with MA expansion
Cytiva has upgraded its Fast Trak facility in Marlborough in response to demand for pre-clinical to Phase II biomanufacturing services from small to mid-sized companies. Under the firm’s Fast Trak contract development and manufacturing brand, Cytiva has expanded its Marlborough cGMP facility to 60,000 square feet. The plant offers biopharma access to services from preclinical to Phase II, including process development, clinical batch manufacturing, analytical development, and QA/QC/regulatory support. Olivier Loeillot, senior vice president of BioProcess at Cytiva, did not…