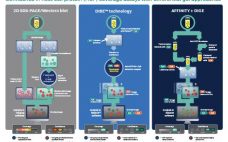
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current…
Tuesday, June 9, 2020 Daily Archives
Achilles becomes fifth company to take residency at CGT Catapult
Achilles Therapeutics will produce clinical material for its personalized T cell candidate from the Cell & Gene Therapy (CGT) Catapult. The Stevenage, UK-based CGT Catapult – established by non-departmental government body Innovate UK in 2017 to support the burgeoning sector – recently completed an expansion, adding six clean rooms. The first company to take manufacturing in this expanded space is Achilles Therapeutics, which is developing autologous cell therapies against advanced non-small cell lung cancer and recurrent metastatic melanoma. Using T…