In this way-too-interesting time we’re living through, we are seeing the worst and best of humanity as we do what we can to protect ourselves and our families. When we can congregate again, some of us will be relieved to abandon home offices and get back to familiar work surroundings, social interactions, dependable Internet access, and company-provided toilet tissue. Others will have learned that working from home is the greatest thing next to sliced bread and lobby with their management…
Sunday, May 24, 2020 Daily Archives
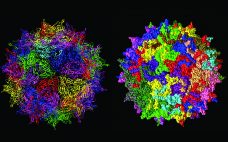
Gene Therapy Trends and Future Prospects
Gene therapy is defined as the transfer of genetic information to a patient for treatment of a disease. Clinical investigation of such therapies began in 1990 with a treatment for a rare immunodeficiency disorder and since has expanded to almost 1,000 clinical studies in 2019 (1, 2). In its most straightforward incarnation, the goal of gene therapy for genetic diseases is long-term expression of a transferred gene at levels that are high enough to be therapeutic, an approach sometimes called…
Cell and Gene Therapies Get a Reality Check: A Conversation with Anthony Davies of Dark Horse Consulting Group
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics — and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week…
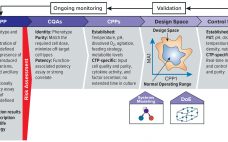
Quality By Design for Advanced Therapies: An Informed Route to Enhanced Late-Stage Clinical Success and Empowered Process Flexibility
As advanced therapies, including regenerative medicines, progress toward commercialization and market approval, early warnings from key opinion leaders (1, 2) regarding the importance of better understanding quality target product profiles (QTPPs) and critical quality attributes (CQAs) of such products have resounded ever louder (see the “Terminology” box for definitions). Costly late-stage delays, redirections, and even abandonment of clinical programs can be linked to quality issues associated with inadequate understanding of process and product. Therefore, a review of the benefits of…
Capture of CH1-Containing Bispecific Antibodies: Evaluating an Alternative to Protein A
Bispecific antibodies (BsAbs) are designed to recognize and bind two different antigens, in many cases for the purpose of immune effector-cell activation to destroy cancer cells (1). Such BsAbs mediate cell killing by binding simultaneously to an antigen that is overexpressed on tumor cells and to the CD3 receptor, activating cytotoxic T lymphocytes (2). Using proprietary UniRat human heavy-chain technology combined with OmniFlic human fixed–light-chain antibody technology licensed from Ligand Pharmaceuticals, Teneobio has produced several bispecific antibodies, each targeting a…
Dynamic Binding Capacities of Protein A Resins for Antibody Capture: A Comparative Evaluation
The dynamic binding capacity (DBC) of a chromatography resin represents the total amount of target protein that the resin will bind under actual flow conditions before significant breakthrough of unbound protein occurs. This is a useful parameter for predicting what the process performance of a resin will be in actual use. DBC affects the overall amount of resin that can be packed in a given column for a process — and the number of batches that can be processed cost-effectively…
A Challenge in Viral Clearance Determination: Estimation of Fifty-Percent Tissue Culture Infective Dose (TCID50) for Low Virus Concentrations
Performing viral clearance studies is an important safety element of manufacturing all biopharmaceuticals expressed from mammalian cells (1). Typically, viral clearance is described as a log reduction value (LRV) and calculated as the log10 of the ratio of input to output virus load. Amounts of virus load are calculated from the volume and concentration of input and output fractions. Virus concentration is often calculated as 50% of tissue-culture infective dose (TCID50) using the Spearman–Kärber (SK) equation (2, 3). In this…
Transforming Personalized Medicine into Off-the-Shelf Cell Therapies
Initial progress in cell and gene therapy has seen 12 advanced therapeutic medicinal products (ATMPs) become available on the market in 2019 for a range of conditions, from monogenic diseases to cancer. Despite such progress, development of clinically and commercially successful cell therapies presents manufacturability challenges and questions about bypassing patients’ immune systems. The availability of rapid sequencing and next-generation bioinformatics has made it possible to understand the mechanisms of disease better and accelerate development of therapeutic responses. The same…
A Rapid, Low-Risk Approach Process Transfer of Biologics from Development to Manufacturing Scale
Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (1). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (2). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according…
Sharing Viral Vector Expertise: A Conversation with Yposkesi’s Chief Executive Officer
As a full-service contract development and manufacturing organization (CDMO) specializing in gene therapy development, Yposkesi produces recombinant adenoassociated virus (AAV) and lentivirus (LV) vectors using adherent-based and suspension-adapted cell expression platforms. Alain Lamproye joined the company as chief executive officer (CEO) in January 2017, having served previously as president of the biopharmaceutical business unit of Novasep (2012–2017) and as CEO of Henogen, its subsidiary dedicated to gene therapy. He has held managerial positions in pharmaceutical operations at Merck Serono (including…