Why is host cell protein (HCP) detection and removal so critical when manufacturing biologics? Product purity and, most importantly, patient safety depends on it. Sandwich ELISA has been a favored method for HCP testing due to its high sensitivity and high throughput, but challenges and limitations exist. Coverage depends strongly on the use of antibody reagents that adequately detect and capture HCPs. In this webinar, we address effective techniques for both optimizing HCP ELISA coverage through the selection of reagents…
Thursday, April 23, 2020 Daily Archives
Oxford University latest horse in COVID-19 race as AAV vaccine enters clinic
The first patients have been dosed with ChAdOx1 nCoV-19, an adenovirus vaccine vector (AAV) developed by the Jenner Institute at the University of Oxford. It becomes the fifth COVID-19 vaccine to enter the clinic. Clinical trials of the vaccine, based on an adenovirus vaccine vector (AAV) and the SARS-CoV-2 spike protein, began today in healthy volunteers aged between 18 – 55 in Oxford, UK. The vaccine was developed at Oxford’s Jenner Institute and was chosen as the most suitable vaccine…
Identification of Host Cell Protein (HCP) Impurities using Antibody Affinity Extraction and Mass Spectrometry Approach
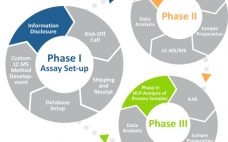
This webcast features: Eric Bishop, Vice President of Research & Development, Cygnus Technologies A well-developed, broadly reactive, and qualified HCP ELISA remains a gold standard method effectively used during the purification process to ensure removal of HCP and to demonstrate process consistency and final drug substance purity. Unfortunately, the ELISA does not inform which HCPs are present or to which HCPs the assay reacts. Mass Spectrometry (MS) is a powerful tool for identifying those HCPs that persist through downstream purification…