As I write, the world is in a state of medical and economic uncertainty related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease (Covid-19) that it causes. Our company group, Informa Connect, has been forced to reschedule some major conferences — including BPI West and BPI Europe — but it’s not alone. Many life-science meetings have been canceled or postponed, and some Biogen employees probably wish their management meeting late in February had been as well.…
Monday, April 13, 2020 Daily Archives
Fighting Alzheimer’s Disease with a Breakthrough Peptide
Even more so than cancer, Alzheimer’s disease is one disease that many people fear greatly. The thought of falling prey to the inevitable desecration of our own minds is something that makes even the bravest among us shudder. If we’re robbed of our sense of who we really are, we imagine, then we are doomed to live our last days without the dignity that defines us and that we hold most dear. The ultimate horror of the condition is that…
A Framework for a Competency-Based Curriculum
Training departments in biopharmaceutical manufacturing facilities are at the forefront of ensuring that their employees are trained in accordance with regulatory compliance standards that govern the industry. More important, the purpose of training is to equip employees with relevant knowledge, skills, and abilities to perform their job functions competently. A competency-based curriculum has the potential to facilitate a training approach that addresses both the practical training needs and desired performance outcomes in a workplace. Competency-Based Curriculum Recently, I was involved…
Shared Clean-in-Place Systems: To Share or Not to Share?
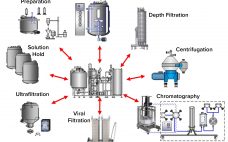
Risk of viral contamination is a an accepted part of developing biopharmaceutical products derived from mammalian-cell culture. Viral safety is achieved through a combination of complementary approaches such as selecting non–animal-derived raw materials, testing cell banks, testing for adventitious virus contamination during cultivation, and demonstrating viral reduction capacity of a purification process (1). The latter commonly is referred to as viral clearance by orthogonal purification. Clearly, viral clearance and appropriate viral segregation are important considerations in biopharmaceutical manufacturing process and…
BVDV Risk Mitigation: Dealing with Bovine Viral Diarrhea Virus in Serum
Bovine serum products such as fetal bovine serum (FBS) are critical, nutrient-rich supplements frequently used in cell culture systems for a number of applications, including biotechnology, animal and human pharmaceutical and diagnostic manufacturing, and life-science research. Serum can be contaminated with adventitious agents that could increase its risk for use in cell culture systems. Bovine viral diarrhea virus (BVDV) is one of the most significant infectious diseases in the livestock industry worldwide because of its high prevalence, strong persistence, and…
Setting a Cornerstone for Platform Purification of Exosomes
Exosomes are a subject of rapidly growing therapeutic interest in the biopharmaceutical industry for two principal reasons. The first reason is that they are the primary communicators of instructions from source cells to target cells. Exosome surface features define their destination. They recognize complementary features on target cells, dock with them, and deliver their programmed instructions in the form of microRNA. The second reason is that exosomes are immunologically silent. As normal human cell products, and by contrast with gene…
Building Orthogonality into Biosimilar Testing
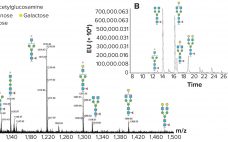
Both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have developed regulatory guidelines on biosimilars (1, 2). These comprehensive documents provide clear guidance on what the agencies expect from structural characterization studies. Using state-of- the-art instrumentation and techniques is expected, but the use of orthogonal techniques in structural comparability assessments is also required. The application of orthogonal analytical techniques will provide a firm structural foundation to claims of biosimilarity. Characterization methods verifying and supporting conclusions drawn…
Brazilian JBS Federally Inspected Fetal Bovine Serum
Serum is the most commonly used supplement in cell culture. Fetal bovine serum (FBS) is the common choice because it contains high concentrations of growth factors and other important signaling molecules (e.g., adhesion proteins, nutrients, carrier proteins, cytokines, and hormones) required for cell survival and differentiation together with its buffering capabilities. FBS production begins with the collection of whole blood from bovine fetuses under aseptic conditions. Once collected, the blood is allowed to clot, and the serum is mechanically separated.…
Developing a Biopharmaceutical Workforce for Today and Tomorrow
Biomanufacturing is constantly evolving, developing new treatments and therapies through cutting-edge methodologies and facing increasing integration of big data and analytics. But keeping up with innovation in this segment requires a workforce with advanced skill sets. The US State of Rhode Island (RI), with a rich manufacturing history and a thriving biotechnology sector, has taken on that challenge. We are preparing a robust talent pool — the greater Providence area contains over 1.6 million people — to become the highly…
Orgenesis signs muscle-derived stem cell pact with Revatis
Orgenesis has added muscle-derived stem cells to its offering through a manufacturing joint venture with Revatis. The partnership will supply developers of autologous cell therapies with exosomes and other cellular products obtained from muscle-derived mesenchymal stem cells (mdMSCs). The firm told us “The objective is to spin-off Revatis technology developed in the vet field into the human field; the JV  is currently planned to be  called “REVACEL. “After technology transfer, REVACEL will conduct development of muscle derived MSCs first and then as sourcing of…