High-throughput technologies have transformed the biotechnology industry. The amount of data they generate is at least a hundred times higher now than it was two decades ago, primarily because of the rise of “-omic” technologies. As in many other industries, the biopharmaceutical sector entered the era of big data the day that high-throughput analytics were routinely implemented in experimental research. Big data refers to “datasets with sizes beyond the ability of commonly used software tools to capture, curate, manage, and…
Thursday, February 6, 2020 Daily Archives
Biopharmaceutical Product Specification Limits and Autocorrelated Data
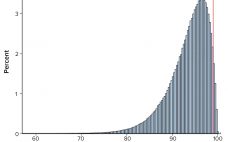
Calculations, including statistical tolerance intervals, can assist in the development and revision of specification acceptance criteria. Manufacturing results for attributes of a biopharmaceutical product can be positively autocorrelated. The sample standard deviation — calculated from limited, positively autocorrelated data — tends to underestimate the long-term process standard deviation (1). In this article, simulated data are used to assess the relative performance of statistical tolerance intervals, intervals calculated using the minimum process performance index Ppk approach, and the sample range. Prevalence…
Bioprocess Development and Qualification: PAT-Based Stage 1 and 2 Acceleration Strategies
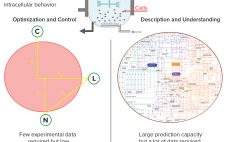
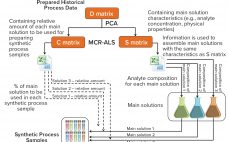
Well-established process analytical technology (PAT) strategies, such as those based on spectroscopy, bring with them several challenges related to the nature of those tools themselves (1–3). Such tools are multiparametric by design — in the sense that most spectroscopies capture multiple attributes sometimes different in nature (e.g., near-infrared, NIR, captures chemical and physical attributes simultaneously). Often a reference method is required; at other times, indirect calibrations are based on the correlation of one culture attribute with another for which a…
Ask the Expert: Highly Sensitive Host-Cell Protein Analyses Using Novel Chromatography Technology
Geert Van Raemdonck (global field support expert at PharmaFluidics) and Koen Sandra (scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (μPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that μPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability…
Ask the Expert: Accelerating Timelines By Integrating Cell-Line Development and Manufacturing
In a 31 October 2019 “Ask the Expert” presentation, Nicole Wakes (group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way…
Ask the Expert: Developing Bioprocesses for Clinical Manufacturing Success
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar, Nigel Shipston (director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development. Shipston’s Presentation The sheer magnitude of investment required…
Ask the Expert: Cell Culture Media Analysis Using Handheld Raman Analyzers
In biopharmaceutical manufacturing, cell culture media supply critical nutrients and maintain pH and osmolality to optimize protein product yield. Because media composition and condition have a strong effect on final biologic product quality and production, biopharmaceutical companies monitor media for lot-to-lot variability. Stability testing for degradation due to light exposure, temperature changes, or shelf-life/time is possible with rapid spectroscopic methods. In an 8 October 2019 “Ask the Expert” webinar, O. Dean Stuart (product manager at Thermo Fisher Scientific) explained how…
Bioengineering for “Benchtop Clinical Trials”
Animal studies can be poor predictors of human drug response. Species-specificity of organs is a concern especially for the heart. Many drugs that enter clinical trials will fail ultimately because of unexpected cardiotoxicity. Drug developers would love to mitigate such risks through in vitro human cardiac testing, but human heart biopsy materials and donor organs do not survive well in a laboratory setting. Breakthroughs with human stem cells offer an alternative. It is now possible to take a simple skin…
Merck’s biosimilar business to power NewCo spinoff
Merck & Co. reported biosimilar sales of $250 million in 2019 and says the revenues will be used to grow its spinout company NewCo, also comprising of its women’s health and legacy brands. Merck & Co. (known as MSD outside North America) announced the creation of NewCo in line with its Q4 and full year 2019 results. The new entity will group the company’s women’s health assets and legacy brands together, along with its biosimilars business. Left alone, Kevin Ali…
Celltrion pulls plant team out of Wuhan as it tracks coronavirus
Celltrion says it is committed to a biosimilars plant in Wuhan, China but has pulled project team out while it tracks the 2019-nCoV coronavirus outbreak. Celltrion is keeping a close eye on the spread of 2019-nCoV according to a spokesman. He told us “while we remain strongly committed to the project, it is difficult to say how the ongoing outbreak will affect our plans at this moment. “We are closely monitoring the situation and will continue to communicate with the…